Oxidation State Of Sulfur In H2so4
News Leon
Mar 23, 2025 · 6 min read
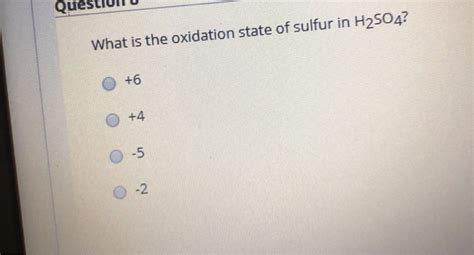
Table of Contents
The Oxidation State of Sulfur in H₂SO₄: A Deep Dive
The seemingly simple question of sulfur's oxidation state in sulfuric acid (H₂SO₄) opens a door to a fascinating exploration of chemical bonding, oxidation-reduction reactions, and the multifaceted nature of this crucial element. This article delves deep into the determination of sulfur's oxidation state in H₂SO₄, exploring different methods and clarifying common misconceptions. We will also discuss the implications of this oxidation state for the chemical properties and reactivity of sulfuric acid.
Understanding Oxidation States
Before diving into the specifics of H₂SO₄, let's establish a firm understanding of what oxidation states represent. The oxidation state, also known as the oxidation number, is a hypothetical charge assigned to an atom in a molecule or ion, assuming that all bonds are completely ionic. It's a crucial concept in redox chemistry, helping us track electron transfer during chemical reactions. While not a true charge, it's a powerful tool for predicting and understanding chemical behavior.
Several rules govern the assignment of oxidation states:
- Free elements: The oxidation state of an atom in its elemental form is always 0. For example, the oxidation state of sulfur in S₈ is 0.
- Monatomic ions: The oxidation state of a monatomic ion equals its charge. For example, the oxidation state of Na⁺ is +1, and Cl⁻ is -1.
- Fluorine: Fluorine, the most electronegative element, always has an oxidation state of -1 in its compounds.
- Hydrogen: Hydrogen generally has an oxidation state of +1, except in metal hydrides (e.g., NaH), where it's -1.
- Oxygen: Oxygen usually has an oxidation state of -2, except in peroxides (e.g., H₂O₂), where it's -1, and in compounds with fluorine (e.g., OF₂), where it's +2.
- Sum of oxidation states: In a neutral molecule, the sum of the oxidation states of all atoms must equal zero. In a polyatomic ion, the sum of the oxidation states must equal the charge of the ion.
Determining the Oxidation State of Sulfur in H₂SO₄
Now, let's apply these rules to determine the oxidation state of sulfur in sulfuric acid (H₂SO₄). We'll use a systematic approach:
-
Hydrogen (H): Each hydrogen atom has an oxidation state of +1. Since there are two hydrogen atoms, the total contribution from hydrogen is +2.
-
Oxygen (O): Each oxygen atom typically has an oxidation state of -2. With four oxygen atoms in H₂SO₄, the total contribution from oxygen is -8.
-
Sulfur (S): Let's represent the oxidation state of sulfur as 'x'.
-
Neutral molecule: The sum of the oxidation states in a neutral molecule must be zero. Therefore, we can set up the equation:
(+2) + x + (-8) = 0
-
Solving for x: Solving this equation, we find that x = +6.
Therefore, the oxidation state of sulfur in H₂SO₄ is +6.
Implications of the +6 Oxidation State
The +6 oxidation state of sulfur in sulfuric acid has profound implications for its chemical properties and reactivity:
-
Strong Acidic Nature: The high oxidation state of sulfur contributes to the strong acidic nature of H₂SO₄. The highly polarized S-O bonds lead to a significant release of protons (H⁺) when H₂SO₄ is dissolved in water, resulting in a highly acidic solution.
-
Oxidizing Agent: Sulfur in the +6 oxidation state is a relatively strong oxidizing agent. This means it can readily accept electrons from other substances, getting reduced in the process while oxidizing the other substance. This oxidizing power is evident in many reactions involving sulfuric acid, such as the oxidation of metals and non-metals. Concentrated sulfuric acid, in particular, is a potent oxidizing agent.
-
Dehydrating Agent: Sulfuric acid's strong affinity for water makes it an excellent dehydrating agent. It can remove water molecules from various substances, leading to the formation of carbon from sugars and the dehydration of alcohols. This property is exploited in numerous industrial applications.
-
Formation of Sulfates: Sulfuric acid readily reacts with metal oxides and hydroxides to form sulfates. These salts, containing the sulfate ion (SO₄²⁻), are widely used in various applications.
Common Misconceptions and Clarifications
Several misconceptions surround the oxidation state of sulfur in sulfuric acid:
-
Ignoring the formal charge: Some may incorrectly assume that the oxidation state is simply the charge on the sulfur atom in a Lewis structure. However, oxidation states are hypothetical charges assigned based on electronegativity, not the actual charges.
-
Incorrect application of rules: Misinterpreting the rules for assigning oxidation states, particularly for hydrogen and oxygen in different contexts, can lead to inaccurate calculations.
-
Overlooking the overall neutrality of the molecule: Failing to consider that the sum of oxidation states in a neutral molecule must be zero is a common error.
It is crucial to follow the systematic rules outlined above to accurately determine the oxidation state of any atom within a molecule.
Further Exploration of Sulfur's Oxidation States
Sulfur is a remarkable element capable of exhibiting a wide range of oxidation states, from -2 to +6. Understanding these different oxidation states is vital for comprehending sulfur's diverse chemistry:
-
Sulfides (-2): In sulfides, such as hydrogen sulfide (H₂S), sulfur exhibits an oxidation state of -2. These compounds are often characterized by their foul odor and reducing properties.
-
Sulfites (+4): Sulfites, containing the sulfite ion (SO₃²⁻), have sulfur in the +4 oxidation state. These compounds are commonly used as preservatives in food and are also reducing agents.
-
Thiosulfates (+2): Thiosulfates, such as sodium thiosulfate (Na₂S₂O₃), contain sulfur in both the +2 and -2 oxidation states. This makes thiosulfate a versatile compound with reducing properties.
-
Disulfides (0): Elemental sulfur (S₈) and disulfides (e.g., dimethyl disulfide) exhibit an oxidation state of 0.
Sulfuric Acid: Industrial Importance and Applications
Sulfuric acid's importance in industry is undeniable. It's a cornerstone chemical used in countless applications, including:
-
Fertilizer Production: A massive portion of sulfuric acid production is dedicated to the manufacturing of fertilizers, particularly ammonium sulfate and superphosphates.
-
Metal Refining: Sulfuric acid plays a crucial role in the refining of metals, such as copper and zinc.
-
Petroleum Refining: It's used in the refining of petroleum products, including the alkylation process for producing high-octane gasoline.
-
Chemical Synthesis: Sulfuric acid serves as a crucial reagent and catalyst in the synthesis of numerous chemicals, including dyes, detergents, and plastics.
-
Battery Production: It is a key component of lead-acid batteries, powering numerous vehicles and devices.
Conclusion
The +6 oxidation state of sulfur in H₂SO₄ is a key factor determining its strong acidic and oxidizing properties. Understanding this oxidation state, along with the rules for assigning oxidation numbers, is crucial for comprehending the chemical behavior of sulfuric acid and its wide range of applications. This article provides a detailed explanation of the determination process and clarifies common misconceptions. The diverse oxidation states of sulfur highlight the element's rich and multifaceted chemistry, with implications spanning various scientific fields and industrial processes. Further exploration of sulfur's chemistry reveals its significant role in both natural and man-made environments.
Latest Posts
Latest Posts
-
In A Eukaryotic Cell Dna Is Found In
Mar 25, 2025
-
A Uniform Solid Sphere Of Radius R
Mar 25, 2025
-
How Many Corners Are On A Cube
Mar 25, 2025
-
Do Birds Have 4 Chambered Heart
Mar 25, 2025
-
One Atomic Mass Unit Is Equal To
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Oxidation State Of Sulfur In H2so4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
