Rutherford Conclusion From Gold Foil Experiment
News Leon
Mar 22, 2025 · 6 min read
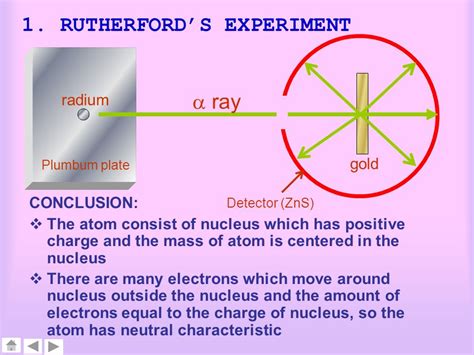
Table of Contents
Rutherford's Gold Foil Experiment: A Revolutionary Conclusion that Reshaped Atomic Theory
Ernest Rutherford's gold foil experiment, conducted in 1909 by Hans Geiger and Ernest Marsden under Rutherford's supervision, stands as a pivotal moment in the history of physics. This seemingly simple experiment, involving the bombardment of a thin gold foil with alpha particles, yielded astonishing results that completely overturned the prevailing plum pudding model of the atom and paved the way for our modern understanding of atomic structure. This article will delve deep into the experiment, its methodology, the surprising results, and, most importantly, the groundbreaking conclusions Rutherford drew from it.
The Prevailing Plum Pudding Model
Before Rutherford's experiment, the accepted model of the atom was the "plum pudding" model proposed by J.J. Thomson. This model depicted the atom as a sphere of positive charge with negatively charged electrons embedded within it, much like plums scattered throughout a pudding. This model, while a significant advancement over previous atomic theories, failed to adequately explain certain phenomena and lacked predictive power.
Limitations of the Plum Pudding Model
The plum pudding model struggled to account for several experimental observations. It couldn't explain the discrete nature of atomic spectra, the specific wavelengths of light emitted by different elements. Moreover, it couldn't account for the scattering of alpha particles, which would become crucial in understanding its shortcomings. The relatively uniform distribution of positive charge predicted by the model couldn't explain the significant deflections observed in Rutherford's experiment.
The Gold Foil Experiment: Methodology and Setup
Rutherford's experiment was remarkably simple in its design but profound in its implications. A beam of alpha particles (positively charged helium nuclei) was directed at a very thin sheet of gold foil, only a few atoms thick. Surrounding the gold foil was a screen coated with zinc sulfide, a substance that scintillates (produces flashes of light) when struck by an alpha particle. The experimental setup allowed the researchers to observe the scattering pattern of the alpha particles after they interacted with the gold foil.
Anticipations and Expectations
Based on the plum pudding model, Rutherford and his team expected the alpha particles to pass through the gold foil with only minor deflections. The relatively diffuse positive charge in the plum pudding model should have resulted in minimal interactions with the positively charged alpha particles. The anticipated scattering pattern was a slight blurring of the alpha particle beam, a minor deviation from a straight path.
The Astonishing Results: Defying Expectations
The experimental results were far from what was expected. While most alpha particles did indeed pass through the gold foil undeflected, a significant number were deflected at large angles, some even bouncing back almost directly towards the source. This observation was completely unexpected and fundamentally challenged the plum pudding model.
Unexpected Large Angle Deflections
The large-angle deflections suggested a concentrated positive charge within the atom, a point of intense repulsive force capable of dramatically altering the trajectory of the alpha particles. This was a revolutionary finding, utterly inconsistent with the diffuse positive charge distribution predicted by the plum pudding model. The fact that some alpha particles bounced back indicated that they had encountered a very strong, localized repulsive force, far beyond what the plum pudding model could account for.
Rutherford's Revolutionary Conclusions: The Nuclear Model
Based on the experimental findings, Rutherford proposed a new model of the atom, the nuclear model. This model posited that:
- The atom is mostly empty space: The fact that most alpha particles passed through the gold foil undeflected indicated that the atom consists largely of empty space.
- The atom has a small, dense, positively charged nucleus: The large-angle deflections of some alpha particles suggested the presence of a small, dense region of positive charge at the center of the atom – the nucleus. This nucleus contains most of the atom's mass.
- Electrons orbit the nucleus: To maintain electrical neutrality, the negatively charged electrons must orbit this positively charged nucleus at a significant distance.
Implications of the Nuclear Model
The nuclear model of the atom revolutionized our understanding of atomic structure. It offered a much more accurate representation of the atom compared to the plum pudding model. It provided a framework for explaining the previously unexplained observations, including the discrete nature of atomic spectra and various other atomic phenomena. The discovery of the atomic nucleus had a profound impact on various fields, including nuclear physics, chemistry, and materials science.
Refinements and Further Developments
Rutherford's nuclear model, while revolutionary, was not without its limitations. It failed to explain the stability of the atom. According to classical physics, an orbiting electron should constantly radiate energy and eventually spiral into the nucleus, collapsing the atom. This problem was addressed later with the development of quantum mechanics and the Bohr model of the atom, which introduced quantized energy levels and addressed the stability issue.
The Bohr Model and Quantum Mechanics
Niels Bohr's model built upon Rutherford's nuclear model by incorporating the principles of quantum mechanics. Bohr proposed that electrons orbit the nucleus in specific energy levels, and they can only transition between these levels by absorbing or emitting photons of specific energies. This successfully explained the discrete nature of atomic spectra, a phenomenon that the Rutherford model couldn't fully address. The subsequent development of quantum mechanics provided a far more complete and accurate description of atomic structure and behavior.
Significance of Rutherford's Gold Foil Experiment
Rutherford's gold foil experiment was a landmark achievement in the history of science. It marked a paradigm shift in our understanding of the atom, transforming our perception from a diffuse, pudding-like structure to a structured system with a dense, positively charged nucleus and orbiting electrons. Its simplicity and elegance belied the profound implications of its results, which laid the foundation for much of modern atomic physics and related fields.
Lasting Impact on Scientific Understanding
The experiment's lasting impact stems from its ability to demonstrate the existence of the atomic nucleus, a concept previously unknown. This discovery opened up entirely new avenues of research in nuclear physics, leading to the development of nuclear power, nuclear medicine, and our understanding of radioactive decay. The experiment also served as a powerful example of how a carefully designed experiment can lead to revolutionary scientific breakthroughs, even when the results deviate significantly from initial expectations.
Conclusion: A Paradigm Shift in Atomic Theory
Rutherford's gold foil experiment stands as a testament to the power of experimental inquiry. Its simple yet ingenious design produced results that overturned existing theories and established a new paradigm in atomic physics. The discovery of the atomic nucleus, the mostly empty nature of the atom, and the resulting nuclear model fundamentally transformed our understanding of matter and laid the groundwork for future advancements in science and technology. The experiment remains a cornerstone of physics education, highlighting the importance of careful observation, rigorous experimentation, and the willingness to challenge established dogma. The legacy of Rutherford's work continues to inspire scientists and researchers to explore the unknown and push the boundaries of human understanding. The conclusions drawn from this seemingly simple experiment remain a profound testament to the power of scientific inquiry and the enduring beauty of scientific discovery.
Latest Posts
Latest Posts
-
Why Does Voltmeter Has High Resistance
Mar 23, 2025
-
Which Of The Following Is A Trinomial
Mar 23, 2025
-
Select The Components Of An Atp Molecule
Mar 23, 2025
-
The Members Of A Homologous Pair Of Chromosomes
Mar 23, 2025
-
A Characteristic Of Human Wants Is That They Are
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Rutherford Conclusion From Gold Foil Experiment . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
