What Reagent Can Affect The Following Transformation
News Leon
Mar 26, 2025 · 6 min read
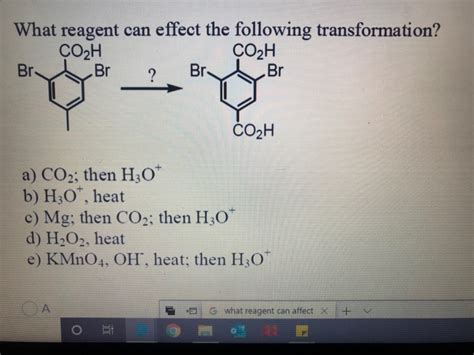
Table of Contents
What Reagent Can Affect the Following Transformation? A Comprehensive Guide
The question "What reagent can affect the following transformation?" is a fundamental one in organic chemistry. It requires a deep understanding of reaction mechanisms, functional group reactivity, and the specific properties of various reagents. This article will delve into this topic, providing a framework for analyzing such problems and exploring numerous examples, showcasing the diversity of reagents and their applications in organic synthesis. We will focus on the strategic use of reagents to achieve specific transformations, highlighting the importance of chemoselectivity, regioselectivity, and stereoselectivity.
Before we jump into specific examples, let's establish a foundational understanding. The choice of reagent depends entirely on the desired transformation. Different functional groups react differently with different reagents. Understanding the reactivity of functional groups is paramount. For example, alcohols can be oxidized, alkyl halides can undergo substitution or elimination, and carbonyl groups can undergo nucleophilic addition or reduction. Furthermore, the reaction conditions (temperature, solvent, pH) play a critical role in determining the outcome of the reaction.
Understanding the Transformation: A Crucial First Step
Before even considering potential reagents, one must thoroughly understand the desired transformation. This involves identifying the starting material, the product, and the specific changes that need to occur. Is it an oxidation, reduction, substitution, addition, elimination, rearrangement, or a combination of these? A clear understanding of the transformation is the bedrock of selecting the appropriate reagent.
Categorizing Reagents Based on Their Functionality:
Reagents can be broadly categorized based on their functionality and the types of transformations they facilitate. Here are some key categories:
1. Oxidizing Agents: These reagents increase the oxidation state of a molecule. Examples include:
- Potassium permanganate (KMnO₄): A strong oxidizing agent capable of oxidizing alcohols to carboxylic acids and alkenes to diols.
- Chromic acid (H₂CrO₄): Another strong oxidizing agent, often used in the Jones oxidation of primary and secondary alcohols.
- Pyridinium chlorochromate (PCC): A milder oxidizing agent that typically oxidizes primary alcohols to aldehydes and secondary alcohols to ketones.
- Dess-Martin periodinane (DMP): A mild and selective oxidizing agent frequently used for the oxidation of primary alcohols to aldehydes.
- Swern oxidation reagents (DMSO, oxalyl chloride): Used for the oxidation of primary and secondary alcohols, particularly sensitive compounds.
2. Reducing Agents: These reagents decrease the oxidation state of a molecule. Examples include:
- Lithium aluminum hydride (LiAlH₄): A powerful reducing agent that reduces a wide range of functional groups, including esters, ketones, aldehydes, and carboxylic acids.
- Sodium borohydride (NaBH₄): A milder reducing agent compared to LiAlH₄, primarily used for the reduction of aldehydes and ketones.
- Diborane (B₂H₆): Often used for the reduction of carboxylic acids to alcohols.
- Hydrogenation catalysts (Pd/C, Pt/C, Ni): Used for the reduction of alkenes and alkynes.
3. Nucleophiles: These reagents possess a lone pair of electrons and are attracted to positively charged or electron-deficient centers. Examples include:
- Grignard reagents (RMgX): Strong nucleophiles that add to carbonyl groups.
- Organolithium reagents (RLi): Similar to Grignard reagents, but generally more reactive.
- Cyanide (CN⁻): A nucleophile that can add to carbonyl groups, forming cyanohydrins.
- Alcohols (ROH): Can act as weak nucleophiles in certain reactions.
4. Electrophiles: These reagents are electron-deficient and are attracted to electron-rich centers. Examples include:
- Alkyl halides (RX): Common electrophiles in substitution and elimination reactions.
- Acyl halides (RCOCl): React with nucleophiles to form esters, amides, and other derivatives.
- Aldehydes and ketones: Can act as electrophiles in nucleophilic addition reactions.
5. Bases: These reagents abstract protons and increase the electron density of a molecule. Examples include:
- Sodium hydroxide (NaOH): A strong base used in many reactions, including saponification and elimination reactions.
- Potassium tert-butoxide (t-BuOK): A strong, sterically hindered base often used in elimination reactions.
- Lithium diisopropylamide (LDA): A strong, non-nucleophilic base used in enolate formation.
6. Acids: These reagents donate protons and can catalyze many reactions. Examples include:
- Sulfuric acid (H₂SO₄): A strong acid used in esterification, dehydration, and other reactions.
- Hydrochloric acid (HCl): A strong acid used in many reactions, including the addition of HCl to alkenes.
- Lewis acids (AlCl₃, BF₃): Electron-deficient compounds that act as catalysts in many reactions.
Specific Examples of Transformations and Suitable Reagents:
Let's consider some specific transformations and the reagents that can affect them:
A. Conversion of an alcohol to an alkyl halide: This transformation involves a substitution reaction. Suitable reagents include:
- Thionyl chloride (SOCl₂): Converts primary and secondary alcohols to alkyl chlorides.
- Phosphorus tribromide (PBr₃): Converts primary and secondary alcohols to alkyl bromides.
- Hydrogen halides (HCl, HBr, HI): Can also convert alcohols to alkyl halides, though often with lower selectivity.
B. Conversion of an alkene to an epoxide: This transformation involves the addition of an oxygen atom across the double bond. A common reagent is:
- Meta-chloroperoxybenzoic acid (mCPBA): A peroxyacid that effectively epoxidizes alkenes.
C. Conversion of an aldehyde to an alcohol: This transformation is a reduction reaction. Suitable reagents include:
- Sodium borohydride (NaBH₄): Reduces aldehydes to primary alcohols.
- Lithium aluminum hydride (LiAlH₄): Also reduces aldehydes to primary alcohols, but is more reactive.
D. Conversion of a carboxylic acid to an ester: This transformation is an esterification reaction. The most common method is:
- Fischer esterification: This reaction employs a carboxylic acid, an alcohol, and an acid catalyst (such as sulfuric acid).
E. Conversion of a ketone to an alkene: This usually involves a Wittig reaction. A key reagent is:
- Wittig reagent: A phosphorus ylide generated from a phosphonium salt.
F. Conversion of an alkyl halide to an alkene: This is an elimination reaction. Common reagents include:
- Strong bases: Such as potassium tert-butoxide (t-BuOK) or sodium ethoxide (NaOEt). These favor elimination over substitution.
Conclusion:
Selecting the appropriate reagent for a specific transformation requires a deep understanding of organic chemistry principles. This article provides a comprehensive overview of various reagent categories and their applications, along with specific examples to illustrate their use. The key to success lies in carefully analyzing the starting material, the desired product, and the specific reaction mechanism involved. Furthermore, considering factors such as reaction conditions and selectivity is crucial for achieving high yields and desired product purity. Continued practice and study of reaction mechanisms are vital for mastering this critical aspect of organic synthesis. Remember, the choice of reagent is dictated by the specific transformation, and understanding the reactivity of functional groups is essential for successful organic synthesis. This overview should provide a robust foundation for tackling more complex transformations and choosing the right reagents for the job. The world of reagents is vast, but with a structured approach and a strong understanding of fundamental principles, navigating this landscape becomes significantly more manageable.
Latest Posts
Latest Posts
-
Is Water A Reactant Or Product
Mar 29, 2025
-
How Many Thousands Make A Lakh
Mar 29, 2025
-
A Slumber Did My Spirit Seal
Mar 29, 2025
-
Letters With A Line Of Symmetry
Mar 29, 2025
-
Marginal Product And Average Product Graph
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Reagent Can Affect The Following Transformation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
