Is Water A Reactant Or Product
News Leon
Mar 29, 2025 · 6 min read
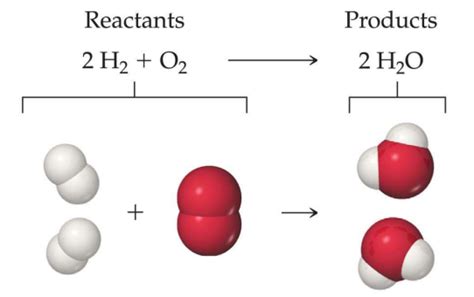
Table of Contents
Is Water a Reactant or Product? Understanding its Role in Chemical Reactions
Water, the elixir of life, plays a multifaceted role in the chemical world. It's far more than just a solvent; it actively participates in numerous reactions, sometimes as a reactant, other times as a product. Understanding its dual nature is crucial to comprehending the intricacies of chemistry. This comprehensive guide will delve into the various scenarios where water acts as either a reactant or a product, providing clear examples and explanations.
Water as a Reactant: The Active Participant
Many chemical reactions wouldn't proceed without water's involvement as a reactant. Its unique properties – polarity, high dielectric constant, and ability to act as both an acid and a base – make it a versatile player in various reaction mechanisms.
1. Hydrolysis Reactions: Breaking Bonds with Water
Hydrolysis, literally meaning "water splitting," is a crucial class of reactions where water molecules are directly involved in breaking chemical bonds. This process is fundamental in numerous biological and industrial processes.
-
Example 1: Ester Hydrolysis: Esters, common in fats and oils, undergo hydrolysis when reacted with water, yielding a carboxylic acid and an alcohol. The water molecule's oxygen atom attacks the carbonyl carbon of the ester, breaking the ester bond and incorporating the water molecule into the products.
-
Example 2: Salt Hydrolysis: Salts of weak acids or bases react with water to form a slightly acidic or basic solution. This happens because water molecules can donate or accept protons (H⁺ ions) from the ions of the salt, altering the pH. For instance, sodium acetate (a salt of a weak acid) reacts with water to produce a slightly basic solution.
-
Example 3: Hydrolysis of ATP: Adenosine triphosphate (ATP), the energy currency of cells, undergoes hydrolysis to release energy. The water molecule breaks a phosphate bond, releasing energy that drives various cellular processes.
2. Hydration Reactions: Adding Water Molecules
Hydration reactions involve the addition of water molecules to a molecule, often changing its structure and properties.
-
Example 1: Hydration of Alkenes: Alkenes, unsaturated hydrocarbons containing a carbon-carbon double bond, can react with water in the presence of an acid catalyst. This reaction, known as acid-catalyzed hydration, adds a water molecule across the double bond, resulting in an alcohol.
-
Example 2: Hydration of Ions: Many ions in aqueous solutions are hydrated, meaning water molecules surround the ions, forming a hydration shell. This process stabilizes the ions and influences their reactivity. The strength of ion-dipole interactions between water molecules and ions plays a vital role in solubility and chemical reactions.
3. Acid-Base Reactions: Water's Amphoteric Nature
Water's amphoteric nature – its ability to act as both an acid (donating a proton) and a base (accepting a proton) – makes it a central player in acid-base chemistry. The autoionization of water, where a water molecule donates a proton to another water molecule, forming hydronium (H₃O⁺) and hydroxide (OH⁻) ions, is a prime example.
-
Example 1: Neutralization Reactions: Acid-base neutralization reactions often involve water as a product, but they can also involve water as a reactant in the sense that the acid or base itself might be diluted and thus be interacting with a large amount of water molecules. For example, a strong acid like HCl will completely dissociate in water, which influences its reactivity with a base.
-
Example 2: Water in Buffer Solutions: Buffer solutions, which resist changes in pH, often involve weak acids or bases and their conjugate salts. Water molecules interact with these species to maintain a relatively constant hydrogen ion concentration.
Water as a Product: The Result of Chemical Reactions
In many chemical reactions, water is formed as a byproduct. This often indicates that the reaction involves the combination of elements or compounds that contain hydrogen and oxygen in a ratio suitable for water formation.
1. Combustion Reactions: Burning and Water Formation
Complete combustion reactions of hydrocarbons (compounds containing only carbon and hydrogen) produce carbon dioxide and water as products. The hydrogen atoms in the hydrocarbon react with oxygen from the air to form water molecules.
-
Example 1: Burning Methane: The combustion of methane (CH₄), the primary component of natural gas, produces carbon dioxide (CO₂) and water (H₂O).
-
Example 2: Burning Ethanol: Ethanol (C₂H₅OH), a common fuel, also produces carbon dioxide and water upon complete combustion.
2. Neutralization Reactions: Acids, Bases, and Water
As mentioned earlier, neutralization reactions between acids and bases often yield water as a product. The hydrogen ions (H⁺) from the acid combine with the hydroxide ions (OH⁻) from the base to form water molecules.
-
Example 1: Reaction of HCl and NaOH: Hydrochloric acid (HCl) reacting with sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H₂O).
-
Example 2: Reaction of H₂SO₄ and KOH: Sulfuric acid (H₂SO₄) reacting with potassium hydroxide (KOH) produces potassium sulfate (K₂SO₄) and water (H₂O).
3. Dehydration Reactions: Removing Water to Form Bonds
Dehydration reactions are the opposite of hydrolysis. In these reactions, water is removed from a molecule, often forming a larger molecule with a new bond.
-
Example 1: Dehydration of Alcohols: Alcohols can undergo dehydration to form alkenes. This reaction involves the removal of a water molecule from the alcohol, creating a carbon-carbon double bond. This requires a catalyst, usually a strong acid like sulfuric acid.
-
Example 2: Formation of Esters: The formation of esters from carboxylic acids and alcohols also involves a dehydration reaction. A water molecule is removed as the ester bond is formed.
4. Redox Reactions: Water's Role in Electron Transfer
Water can be both a reactant and a product in redox reactions. It can act as an oxidizing agent, accepting electrons, or as a reducing agent, donating electrons, depending on the specific reaction conditions.
-
Example 1: Electrolysis of Water: The electrolysis of water involves using electricity to split water molecules into hydrogen and oxygen gas. Water acts as a reactant in this process.
-
Example 2: Photosynthesis: In photosynthesis, water is oxidized, releasing electrons that are used to reduce carbon dioxide into glucose. Water is thus a reactant here. Oxygen gas is a byproduct.
Distinguishing Water's Role: A Closer Look
Determining whether water is a reactant or a product requires a careful examination of the reaction mechanism. In hydrolysis reactions, water actively participates in breaking bonds, clearly establishing its role as a reactant. In contrast, in combustion or neutralization reactions, water is formed as a result of the reaction, making it a product. Dehydration reactions illustrate the opposite process: water is removed, establishing it implicitly as a reactant.
In many cases, it's not strictly "either/or." Water's amphoteric nature and its role as a solvent influence reaction kinetics and equilibrium, even when it’s not directly involved in bond breaking or formation. It’s also crucial to recognize that the context of the reaction, including the presence of catalysts and reaction conditions, profoundly impacts water’s role.
Conclusion: Water's Ubiquitous Importance
Water's role in chemical reactions extends far beyond its simple presence as a solvent. It acts as both a reactant and a product in a vast array of reactions, demonstrating its essential role in various chemical processes occurring in nature and within the laboratory. By understanding water’s multifaceted contributions, we gain a deeper appreciation for its central role in the intricate dance of chemical transformations. Further exploration of specific reaction mechanisms will undoubtedly reveal more of its dynamic and crucial contributions to the chemical world.
Latest Posts
Latest Posts
-
An Example Of A Transfer Payment Is
Apr 01, 2025
-
4 Right Angles And 4 Equal Sides
Apr 01, 2025
-
How Do The Daughter Cells Compare To The Parent Cell
Apr 01, 2025
-
A Group Of Closely Related Species Is A
Apr 01, 2025
-
Which Of The Is Not A Greenhouse Gas
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Water A Reactant Or Product . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
