What Monosaccharide Is Found In Cellulose Starch And Glycogen
News Leon
Mar 17, 2025 · 5 min read
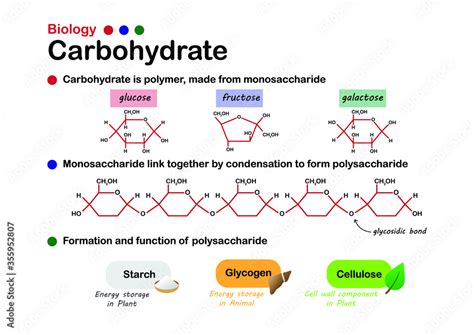
Table of Contents
What Monosaccharide is Found in Cellulose, Starch, and Glycogen?
Polysaccharides are complex carbohydrates composed of long chains of monosaccharides, the simplest form of sugar. Understanding the specific monosaccharide building blocks of these polysaccharides is crucial to comprehending their diverse biological functions. This article delves deep into the monosaccharide composition of cellulose, starch, and glycogen – three vital polysaccharides with distinct structures and roles in living organisms.
The Monosaccharide Foundation: Glucose
The central theme uniting cellulose, starch, and glycogen is their reliance on a single monosaccharide: glucose. While they share this common building block, the way glucose units are linked and arranged dramatically impacts their properties and functions. Think of it like building blocks – you can use the same blocks to build a tower, a wall, or a bridge, each with a vastly different structure and purpose.
Glucose: A Closer Look
Glucose (C₆H₁₂O₆) is an aldohexose, meaning it's a six-carbon sugar with an aldehyde functional group. It exists in two main cyclic forms: α-glucose and β-glucose, which differ only in the orientation of the hydroxyl group (-OH) on carbon atom number 1. This seemingly minor difference has monumental consequences for the resulting polysaccharide.
Isomers and Their Impact: The difference between α-glucose and β-glucose is a prime example of isomerism. Isomers are molecules with the same chemical formula but different structural arrangements. This subtle structural variation significantly influences the properties of the resulting polymers.
Cellulose: The Structural Polysaccharide
Cellulose, the most abundant organic polymer on Earth, is the primary structural component of plant cell walls. It provides rigidity and support to plants, much like the steel framework of a building.
β-1,4-Glycosidic Bonds: The Key to Cellulose Structure
Cellulose is composed of long, unbranched chains of β-glucose molecules linked together by β-1,4-glycosidic bonds. These bonds connect the carbon atom 1 of one β-glucose molecule to the carbon atom 4 of the adjacent β-glucose molecule. This specific linkage results in a linear, rigid structure.
Hydrogen Bonding: Stability and Strength: The linear chains of cellulose are further stabilized by extensive hydrogen bonding between adjacent chains. This extensive network of hydrogen bonds contributes significantly to the immense strength and insolubility of cellulose. This is why cellulose forms strong fibers, providing structural support to plants. This also explains why humans cannot digest cellulose; our digestive enzymes lack the capacity to break down these strong β-1,4-glycosidic bonds.
Cellulose's Significance in Nature and Industry
Cellulose's role extends beyond plant structure. It is a crucial component of many industrial processes:
- Paper production: Cellulose fibers are the primary raw material used in paper manufacturing.
- Textiles: Cellulose is used to produce various textiles, including cotton and rayon.
- Biofuels: Cellulose is being explored as a sustainable source of biofuels.
Starch: The Energy Storage Polysaccharide in Plants
Starch serves as the primary energy storage polysaccharide in plants. It's a readily accessible source of glucose that plants can utilize when needed.
α-1,4 and α-1,6-Glycosidic Bonds: Branching and Energy Storage
Unlike cellulose, starch is composed primarily of α-glucose molecules. These α-glucose units are linked together by α-1,4-glycosidic bonds, forming linear chains called amylose. However, starch also contains branched chains called amylopectin. These branches are formed by α-1,6-glycosidic bonds connecting glucose units at carbon atom 1 and carbon atom 6.
Amylose vs. Amylopectin: Amylose is a linear chain, while amylopectin is highly branched. This branching in amylopectin increases the number of non-reducing ends, which facilitates the rapid breakdown of starch into glucose molecules when energy is needed. This efficient energy release is crucial for plant metabolism.
Starch's Importance in Human Nutrition
Starch is a major source of energy in the human diet. Our digestive system contains enzymes that efficiently break down the α-1,4 and α-1,6 glycosidic bonds in starch, releasing glucose for energy production. Common starch sources include potatoes, rice, wheat, corn, and other grains.
Glycogen: The Energy Storage Polysaccharide in Animals
Glycogen is the primary energy storage polysaccharide in animals, playing a similar role to starch in plants. It's stored primarily in the liver and muscles.
Structure Similar to Amylopectin: High Branching for Rapid Glucose Release
Glycogen's structure closely resembles that of amylopectin, with chains of α-glucose molecules linked by α-1,4-glycosidic bonds and branches formed by α-1,6-glycosidic bonds. However, glycogen is even more highly branched than amylopectin, resulting in a more compact structure.
Rapid Mobilization of Glucose: This extensive branching in glycogen allows for the rapid mobilization of glucose when the body needs energy. The numerous non-reducing ends provide numerous points for enzymatic action, ensuring a swift release of glucose molecules to fuel cellular processes.
Glycogen's Crucial Role in Maintaining Blood Glucose Levels
Glycogen stored in the liver plays a critical role in maintaining stable blood glucose levels. When blood glucose levels drop, the liver releases glucose from glycogen stores to replenish the supply, preventing hypoglycemia (low blood sugar).
Summary Table: Comparing Cellulose, Starch, and Glycogen
| Feature | Cellulose | Starch (Amylose & Amylopectin) | Glycogen |
|---|---|---|---|
| Monosaccharide | β-Glucose | α-Glucose | α-Glucose |
| Glycosidic Bond | β-1,4-glycosidic | α-1,4-glycosidic, α-1,6-glycosidic (branches) | α-1,4-glycosidic, α-1,6-glycosidic (highly branched) |
| Structure | Linear, unbranched | Linear (amylose), branched (amylopectin) | Highly branched |
| Function | Structural support in plants | Energy storage in plants | Energy storage in animals |
| Digestibility (Humans) | Indigestible | Digestible | Digestible |
Conclusion: The Power of Glucose Linkage
The examples of cellulose, starch, and glycogen powerfully illustrate how a single monosaccharide, glucose, can form diverse polysaccharides with vastly different properties and functions simply by altering the type of glycosidic bond. The seemingly small difference between α- and β-glucose profoundly impacts the structure and consequently the biological roles of these essential polysaccharides. Understanding this fundamental relationship is crucial for appreciating the intricate complexity and efficiency of biological systems. Furthermore, this knowledge is vital in fields ranging from food science and nutrition to materials science and biofuel research.
Latest Posts
Latest Posts
-
Find The Current Through The 12 O Resistor
Mar 18, 2025
-
During The Civil War Southern Leaders Hoped That
Mar 18, 2025
-
Which Of The Following Is Not Correctly Matched
Mar 18, 2025
-
Balanced Equation Of H2so4 And Naoh
Mar 18, 2025
-
A Set That Contains No Elements Is Called The
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Monosaccharide Is Found In Cellulose Starch And Glycogen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
