Balanced Equation Of H2so4 And Naoh
News Leon
Mar 18, 2025 · 6 min read
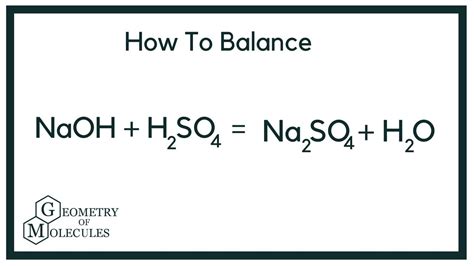
Table of Contents
The Balanced Equation of H₂SO₄ and NaOH: A Deep Dive into Acid-Base Reactions
The reaction between sulfuric acid (H₂SO₄) and sodium hydroxide (NaOH) is a classic example of a strong acid-strong base neutralization reaction. Understanding this reaction, including its balanced equation and the stoichiometry involved, is fundamental to grasping key concepts in chemistry, particularly acid-base chemistry and titration. This comprehensive guide will explore the reaction in detail, covering various aspects from balancing the equation to its applications and implications.
Understanding the Reactants: H₂SO₄ and NaOH
Before delving into the reaction itself, let's briefly review the properties of the individual reactants:
Sulfuric Acid (H₂SO₄)
Sulfuric acid is a strong diprotic acid, meaning it can donate two protons (H⁺ ions) per molecule. It's highly corrosive and is one of the most important industrial chemicals, used extensively in various processes, from fertilizer production to petroleum refining. Its strong acidic nature is due to its complete dissociation in water:
H₂SO₄(aq) → 2H⁺(aq) + SO₄²⁻(aq)
This complete dissociation is what characterizes a strong acid – it essentially releases all its protons when dissolved in water.
Sodium Hydroxide (NaOH)
Sodium hydroxide, also known as caustic soda or lye, is a strong monoprotic base. It's also highly corrosive. It readily dissociates in water, releasing one hydroxide ion (OH⁻) per molecule:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
The presence of the hydroxide ion is what confers its basic properties.
Balancing the Equation: A Step-by-Step Approach
The reaction between H₂SO₄ and NaOH is a neutralization reaction, where the acid and base react to form water and a salt. The balanced equation accurately represents the stoichiometry of this reaction, ensuring the number of atoms of each element is the same on both the reactant and product sides.
The unbalanced equation is:
H₂SO₄(aq) + NaOH(aq) → Na₂SO₄(aq) + H₂O(l)
Notice that the number of sodium (Na), sulfur (S), oxygen (O), and hydrogen (H) atoms are not balanced. To balance this equation, we need to adjust the stoichiometric coefficients:
Step 1: Balancing Sodium (Na)
There are two sodium atoms on the product side (Na₂SO₄) and only one on the reactant side. To balance this, we add a coefficient of 2 in front of NaOH:
H₂SO₄(aq) + 2NaOH(aq) → Na₂SO₄(aq) + H₂O(l)
Step 2: Balancing Hydrogen (H)
Now we have four hydrogen atoms on the reactant side (2 from H₂SO₄ and 2 from 2NaOH) and only two on the product side. Adding a coefficient of 2 in front of H₂O balances the hydrogen atoms:
H₂SO₄(aq) + 2NaOH(aq) → Na₂SO₄(aq) + 2H₂O(l)
Step 3: Checking for Balance
Let's verify the balance:
- Hydrogen (H): 4 on both sides
- Sodium (Na): 2 on both sides
- Sulfur (S): 1 on both sides
- Oxygen (O): 6 on both sides
The equation is now balanced. The final balanced equation is:
H₂SO₄(aq) + 2NaOH(aq) → Na₂SO₄(aq) + 2H₂O(l)
Understanding the Products: Na₂SO₄ and H₂O
The products of the reaction are sodium sulfate (Na₂SO₄) and water (H₂O):
Sodium Sulfate (Na₂SO₄)
Sodium sulfate is a salt – a compound formed from the reaction of an acid and a base. It's a soluble ionic compound, meaning it readily dissolves in water, dissociating into its constituent ions:
Na₂SO₄(aq) → 2Na⁺(aq) + SO₄²⁻(aq)
It has various industrial applications, including in the manufacture of detergents and paper.
Water (H₂O)
Water is formed as a result of the combination of H⁺ ions from the acid and OH⁻ ions from the base:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This is the essence of a neutralization reaction – the formation of water molecules.
Stoichiometry and Calculations
The balanced equation provides the stoichiometric ratios between the reactants and products. For example, the equation indicates that one mole of H₂SO₄ reacts with two moles of NaOH to produce one mole of Na₂SO₄ and two moles of H₂O. This allows us to perform various stoichiometric calculations, such as determining the amount of product formed from a given amount of reactant or vice versa.
Example: If 10 grams of H₂SO₄ react with excess NaOH, how many grams of Na₂SO₄ are produced?
This requires several steps, involving molar masses and mole ratios:
-
Convert grams of H₂SO₄ to moles: Find the molar mass of H₂SO₄ (approximately 98 g/mol). Moles of H₂SO₄ = (10 g) / (98 g/mol) = 0.102 moles.
-
Use the mole ratio: According to the balanced equation, 1 mole of H₂SO₄ produces 1 mole of Na₂SO₄. Therefore, 0.102 moles of H₂SO₄ will produce 0.102 moles of Na₂SO₄.
-
Convert moles of Na₂SO₄ to grams: Find the molar mass of Na₂SO₄ (approximately 142 g/mol). Grams of Na₂SO₄ = (0.102 moles) * (142 g/mol) = 14.5 g (approximately).
Therefore, approximately 14.5 grams of Na₂SO₄ would be produced.
Titration and Applications
The reaction between H₂SO₄ and NaOH is frequently used in titration experiments. Titration is a quantitative analytical technique used to determine the concentration of an unknown solution (analyte) by reacting it with a solution of known concentration (titrant). In this case, we could use a standardized NaOH solution to titrate an unknown solution of H₂SO₄, using an indicator to determine the equivalence point (the point where the moles of acid and base are equal). This allows for accurate determination of the concentration of the sulfuric acid.
Beyond titrations, this reaction finds application in various industrial processes where neutralization is crucial. For example, it's used to neutralize waste streams containing sulfuric acid, ensuring environmental compliance.
Safety Precautions
Both H₂SO₄ and NaOH are highly corrosive substances. Appropriate safety precautions must be taken when handling these chemicals, including wearing protective gear (gloves, goggles, lab coat) and working in a well-ventilated area. Always add acid to water, never water to acid, to prevent splashing and potential burns. Proper disposal procedures should also be followed.
Further Exploration
The reaction between H₂SO₄ and NaOH provides a strong foundation for understanding acid-base chemistry. Further exploration could involve investigating the effects of temperature and concentration on the reaction rate, conducting experimental titrations, or exploring other neutralization reactions involving different acids and bases. The principles learned here are applicable to a wide range of chemical reactions and processes.
This detailed explanation covers the balanced equation of H₂SO₄ and NaOH extensively, including its context, balancing, stoichiometry, applications, and safety considerations. This in-depth approach aims for comprehensive understanding and high search engine optimization.
Latest Posts
Latest Posts
-
Word That Has Two Different Meanings
Mar 18, 2025
-
Is Soil A Homogeneous Or Heterogeneous Mixture
Mar 18, 2025
-
What Does Isinstance Do In Python
Mar 18, 2025
-
What Is The Difference Between Political Parties And Interest Groups
Mar 18, 2025
-
Electromagnetic Radiation At Its Maximum Wavelength Is
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation Of H2so4 And Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
