What Is The Stationary Phase For Paper Chromatography One-word Answer
News Leon
Mar 29, 2025 · 6 min read
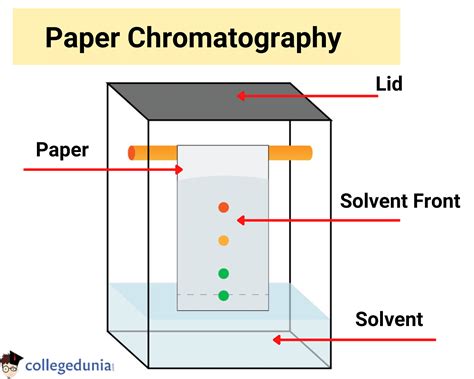
Table of Contents
What is the Stationary Phase for Paper Chromatography? (One-Word Answer: Paper)
Paper chromatography is a simple yet powerful analytical technique used to separate components of a mixture based on their differential affinities for a stationary and a mobile phase. While the question asks for a single-word answer – Paper – understanding the intricacies of this stationary phase is crucial to mastering the technique. This article delves deep into the properties and role of paper as the stationary phase in paper chromatography, exploring its composition, interactions with the analyte, and its impact on separation efficiency.
Understanding the Stationary Phase in Chromatography
Chromatography, in general, relies on the differential partitioning of components between two phases: a stationary phase and a mobile phase. The stationary phase remains fixed, while the mobile phase moves over it, carrying the mixture's components. The components interact differently with both phases, leading to their separation as they travel at different rates. The more strongly a component interacts with the stationary phase, the slower it moves. Conversely, components with a higher affinity for the mobile phase travel faster.
In paper chromatography, the stationary phase is the paper itself. However, it's not just any paper; the specific properties of the paper play a vital role in the separation process.
The Composition and Properties of Paper as a Stationary Phase
The paper used in paper chromatography is typically made of cellulose fibers. Cellulose is a polysaccharide composed of glucose units linked together to form long chains. These chains are interwoven to create the paper's structure. The hydroxyl (-OH) groups present on the glucose units are critical to the paper's function as a stationary phase. These hydroxyl groups are highly polar and capable of forming hydrogen bonds with polar molecules. This capability is fundamental to the separation mechanism.
Several characteristics of the paper influence its performance as a stationary phase:
-
Porosity: The paper's porous nature allows the mobile phase to move through it, carrying the sample components. The pore size and distribution impact the rate of mobile phase flow and, subsequently, the separation efficiency. A well-defined pore structure ensures uniform solvent flow, preventing uneven separation.
-
Water Retention: Cellulose fibers readily absorb water. This adsorbed water forms a thin layer on the paper's surface and within its pores, acting as a crucial part of the stationary phase. This thin layer of water interacts with the sample components through hydrogen bonding and other intermolecular forces. The amount of water retained by the paper (its water content) significantly affects the separation process. A higher water content can lead to better separation of polar compounds, while lower water content might be preferable for less polar substances.
-
Purity: High-purity cellulose paper is essential to minimize unwanted interactions between the sample components and impurities in the paper. Impurities could interfere with the separation process, leading to inaccurate results.
-
Thickness: The paper's thickness also affects the separation. Thicker paper offers a longer path for the mobile phase and sample components to travel, potentially leading to better separation. However, the separation time may increase with thicker paper.
-
Type of Paper: Different types of paper (e.g., filter paper, chromatography paper) have different properties affecting the separation process. Chromatography paper is specifically designed for optimal performance in chromatography, while filter paper might not be as suitable due to its varying properties.
The Mechanism of Separation in Paper Chromatography
The separation in paper chromatography arises from the interplay of several forces:
-
Adsorption: Sample components can adsorb onto the surface of the cellulose fibers or the adsorbed water layer. The strength of adsorption depends on the polarity of the components and the paper's surface. Polar compounds tend to adsorb more strongly than nonpolar ones.
-
Partitioning: The sample components partition between the mobile phase and the stationary phase (the water layer on the cellulose). The distribution coefficient (Kd) defines the ratio of the concentration of a component in the stationary phase to its concentration in the mobile phase. A high Kd indicates a strong interaction with the stationary phase, leading to slower migration.
-
Capillary Action: The capillary action of the paper draws the mobile phase upwards, carrying the sample components with it. The rate of movement of each component is dictated by its differential affinity for the stationary and mobile phases.
Optimization of Paper Chromatography
Several factors can be optimized to enhance the separation efficiency in paper chromatography:
-
Choice of Solvent (Mobile Phase): Selecting an appropriate solvent system is crucial. The solvent's polarity should be carefully chosen to match the polarity of the compounds being separated. A mixture of solvents can provide better separation than a single solvent.
-
Temperature: Temperature affects the solvent viscosity and the solubility of the components. A constant temperature is essential for reproducible results.
-
Sample Preparation: Proper sample preparation is essential. The sample should be dissolved in a suitable solvent and applied as a small, concentrated spot to the paper.
-
Paper Quality: Using high-quality chromatography paper ensures reproducible results and minimizes the influence of impurities.
-
Developing Technique: Various developing techniques, like ascending or descending chromatography, can be employed to optimize separation.
Applications of Paper Chromatography
Paper chromatography has numerous applications in various fields, including:
-
Analytical Chemistry: Separating and identifying components in mixtures, especially in qualitative analysis.
-
Biochemistry: Analyzing amino acids, sugars, and other biomolecules.
-
Forensic Science: Analyzing inks, dyes, and other substances found at crime scenes.
-
Pharmaceutical Industry: Analyzing drug formulations and impurities.
-
Environmental Science: Monitoring pollutants in water and soil samples.
Limitations of Paper Chromatography
While paper chromatography is a simple and inexpensive technique, it has certain limitations:
-
Lower Resolution: Compared to more advanced techniques like HPLC, it offers lower resolution, limiting its effectiveness in separating complex mixtures.
-
Slower Separation: The separation process is relatively slow, often requiring several hours.
-
Qualitative Analysis: It's primarily suitable for qualitative analysis, although quantitative analysis is possible with careful calibration.
-
Sensitivity: The sensitivity can be lower compared to other chromatographic techniques.
Conclusion
In summary, the stationary phase in paper chromatography is paper itself, specifically the cellulose fibers and the adsorbed water layer. Its porous structure, water retention capacity, and the hydroxyl groups on the cellulose enable the separation of components based on their differential interactions with the stationary and mobile phases. Understanding the characteristics of the paper and optimizing the experimental conditions is essential for achieving effective separation. While it might have some limitations, paper chromatography remains a valuable tool for qualitative analysis and educational purposes due to its simplicity, cost-effectiveness, and ease of implementation. Its historical importance and continued use in educational settings underscore its lasting relevance in the realm of analytical chemistry.
Latest Posts
Latest Posts
-
Which Of The Following Is The Site Of Translation
Apr 01, 2025
-
What Is The Atomic Number Of An Atom Equal To
Apr 01, 2025
-
Parathyroid Hormone Does All Of The Following Except
Apr 01, 2025
-
Which Structure Is Not Part Of A Neuron
Apr 01, 2025
-
An Asset Created By Prepayment Of An Insurance Premium Is
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Stationary Phase For Paper Chromatography One-word Answer . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
