What Is The Atomic Number Of An Atom Equal To
News Leon
Apr 01, 2025 · 6 min read
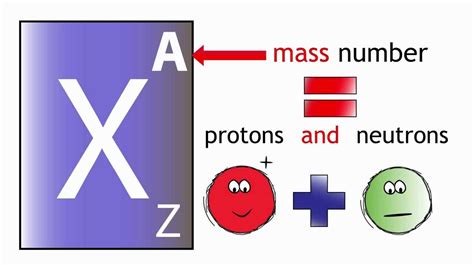
Table of Contents
What is the Atomic Number of an Atom Equal To? A Deep Dive into Atomic Structure and Properties
The atomic number of an atom is a fundamental concept in chemistry and physics, crucial for understanding the behavior and properties of elements. It's a seemingly simple number, yet it holds immense significance, dictating an atom's identity and its interactions with other atoms. This article delves deep into the meaning of atomic number, its relationship to other atomic properties, and its implications across various scientific fields.
Understanding the Atomic Number: More Than Just a Number
The atomic number (Z) of an atom is equal to the number of protons found in the atom's nucleus. This is a defining characteristic, uniquely identifying each element in the periodic table. For example, hydrogen (H) has an atomic number of 1, meaning it possesses one proton in its nucleus. Helium (He), with an atomic number of 2, has two protons. This simple numerical value unlocks a wealth of information about an atom's structure and behavior.
Why is the Atomic Number So Important?
The atomic number's importance stems from several key reasons:
-
Elemental Identity: The atomic number definitively identifies an element. No two elements share the same atomic number. This is the basis for organizing the periodic table, arranging elements based on increasing atomic number and reflecting their recurring chemical properties.
-
Number of Electrons (in Neutral Atoms): In a neutral atom, the number of protons in the nucleus is equal to the number of electrons orbiting the nucleus. This balance of positive and negative charges results in an electrically neutral atom. The electrons determine an atom's chemical behavior and its ability to form chemical bonds with other atoms.
-
Chemical Properties: The arrangement of electrons, determined by the number of protons (atomic number), governs an atom's chemical properties. Elements in the same column of the periodic table (groups or families) have similar electron configurations in their outermost shell, leading to similar chemical reactivities.
-
Isotopes and Atomic Mass: While the atomic number defines the element, isotopes of the same element can exist. Isotopes have the same number of protons (and thus the same atomic number) but differ in the number of neutrons. The atomic mass of an element is an average of the masses of its isotopes, weighted by their relative abundances.
Delving Deeper into Atomic Structure and its Relation to Atomic Number
To fully grasp the significance of the atomic number, we must understand the structure of an atom. Atoms are composed of three fundamental subatomic particles:
-
Protons: Positively charged particles residing in the atom's nucleus. The number of protons determines the atomic number (Z).
-
Neutrons: Neutral particles (no charge) also located in the nucleus. The number of neutrons can vary within isotopes of the same element, affecting the atom's mass.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons in a neutral atom equals the number of protons.
Electron Shells and Chemical Reactivity
The arrangement of electrons in electron shells is crucial for understanding an atom's chemical behavior. Electrons occupy specific energy levels, and the outermost shell, known as the valence shell, plays a particularly important role in chemical bonding. Atoms tend to react with other atoms to achieve a stable electron configuration, often a full valence shell. This drive for stability explains why atoms form chemical bonds.
The number of valence electrons is directly related to the atomic number and the element's position in the periodic table. For example, elements in Group 1 (alkali metals) have one valence electron, making them highly reactive. Elements in Group 18 (noble gases) have a full valence shell, resulting in their inertness.
Isotopes: Same Atomic Number, Different Mass
Isotopes are atoms of the same element that share the same atomic number (same number of protons) but differ in their number of neutrons. This difference in neutron number leads to variations in the atom's mass. For example, carbon-12 (¹²C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons. Both are isotopes of carbon, both have an atomic number of 6, but they have different masses.
The existence of isotopes has important implications in various fields:
-
Radioactive Isotopes: Some isotopes are radioactive, meaning their nuclei are unstable and decay over time, emitting radiation. Radioactive isotopes have many applications, including medical imaging, carbon dating, and cancer treatment.
-
Nuclear Chemistry: The study of nuclear reactions and transformations involves understanding isotopes and their properties.
-
Geochemistry and Environmental Science: Isotope ratios can be used to trace the origin and movement of materials in the environment, providing insights into geological processes and environmental changes.
The Atomic Number and the Periodic Table: A Perfect Match
The periodic table is a powerful tool for organizing elements based on their atomic numbers and recurring chemical properties. The table arranges elements in order of increasing atomic number, reflecting their properties and trends.
Periodic Trends and Atomic Number
Several important periodic trends are directly related to the atomic number:
-
Atomic Radius: Atomic radius generally increases down a group (column) as the number of electron shells increases. It generally decreases across a period (row) due to increasing nuclear charge.
-
Ionization Energy: The energy required to remove an electron from an atom generally increases across a period and decreases down a group.
-
Electronegativity: The tendency of an atom to attract electrons in a chemical bond generally increases across a period and decreases down a group.
The Atomic Number in Various Scientific Fields
The atomic number plays a critical role in numerous scientific disciplines beyond basic chemistry:
-
Nuclear Physics: The atomic number is fundamental in understanding nuclear reactions, including fission and fusion. The stability of nuclei is influenced by the ratio of protons to neutrons.
-
Astrophysics: The abundance of elements in stars and other celestial bodies is linked to their atomic numbers and nuclear processes. Spectroscopic analysis of starlight allows astronomers to determine the elemental composition of distant objects based on the atomic numbers of their constituent atoms.
-
Materials Science: The atomic number and electronic structure of elements determine the properties of materials, influencing their conductivity, strength, and reactivity. Materials scientists leverage this understanding to develop new materials with desired properties.
-
Medical Science: Radioactive isotopes, identified by their atomic number, are used extensively in medical imaging techniques such as PET scans and various therapeutic applications.
-
Environmental Science: Isotope analysis based on atomic number helps monitor pollution levels, trace pollutants' sources and track the movement of contaminants in the environment.
Conclusion: The Significance of a Simple Number
The atomic number, though a seemingly simple numerical value, represents a cornerstone of our understanding of the atom and its behavior. Its profound implications span diverse scientific fields, from fundamental chemistry to advanced nuclear physics and astrophysics. It serves as the definitive identifier of an element and the key to unlocking its properties and interactions. Understanding the atomic number is not merely knowing a number; it is grasping the essence of matter itself and its organization in the universe. Its importance extends far beyond the classroom and is vital for progress in a wide array of scientific and technological endeavors.
Latest Posts
Latest Posts
-
Are Metals Solid At Room Temperature
Apr 02, 2025
-
Which Of The Following Statements Correctly Describes Gene Linkage
Apr 02, 2025
-
Complete The Complementary Strand Of Dna
Apr 02, 2025
-
Is Carbon Dioxide A Pure Substance Or Mixture
Apr 02, 2025
-
The Given Reaction Proceeds In Two Parts
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Atomic Number Of An Atom Equal To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
