What Is The Specific Heat Capacity Of Aluminum
News Leon
Mar 19, 2025 · 7 min read
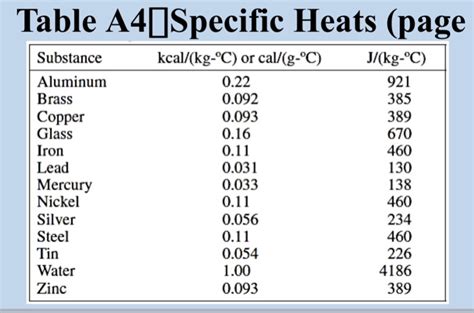
Table of Contents
What is the Specific Heat Capacity of Aluminum? A Deep Dive
Aluminum, a lightweight yet remarkably strong metal, finds applications across diverse industries, from aerospace engineering to kitchenware. Understanding its thermal properties, specifically its specific heat capacity, is crucial for optimizing its use in various applications. This article delves into the intricacies of aluminum's specific heat capacity, exploring its definition, measurement, factors influencing it, and its significance in different contexts.
Defining Specific Heat Capacity
Specific heat capacity, often simply referred to as specific heat, is a fundamental physical property that quantifies the amount of heat energy required to raise the temperature of one unit of mass of a substance by one degree Celsius (or one Kelvin). It's a measure of a substance's resistance to temperature change. A high specific heat capacity indicates that a substance can absorb a significant amount of heat energy with a relatively small temperature increase, while a low specific heat capacity means the opposite. This property is crucial in various engineering and scientific applications.
The specific heat capacity is usually denoted by the symbol 'c' and is expressed in units of Joules per kilogram-kelvin (J/kg·K) or Joules per gram-celsius (J/g·°C). These units reflect the energy (Joules) needed to change the temperature of a specific mass (kilograms or grams) by one degree.
Specific Heat Capacity of Aluminum: The Value
The specific heat capacity of aluminum is approximately 900 J/kg·K or 0.9 J/g·°C. It's important to note that this value can slightly vary depending on factors such as the purity of the aluminum, its crystalline structure, and the temperature range considered. However, 900 J/kg·K serves as a reliable approximation for most practical purposes. This relatively high specific heat capacity compared to many other metals signifies that aluminum can absorb and release a considerable amount of heat before experiencing a substantial temperature change.
Why is the Specific Heat Capacity of Aluminum Important?
The high specific heat capacity of aluminum has several practical implications:
-
Heat sinks: Aluminum's ability to absorb significant heat without experiencing a dramatic temperature rise makes it an ideal material for heat sinks in electronic devices. Heat sinks dissipate heat generated by components, preventing overheating and ensuring efficient operation. The higher the specific heat capacity, the better the heat sink can absorb the heat produced, maintaining lower operating temperatures.
-
Cookware: Aluminum's high specific heat capacity, coupled with its excellent thermal conductivity, makes it a popular choice for cookware. It heats up quickly and evenly, ensuring consistent cooking and minimizing hot spots. This is especially beneficial for applications requiring precise temperature control.
-
Automotive applications: Aluminum alloys are widely used in automotive parts due to their lightweight nature and good thermal properties. Their ability to absorb heat efficiently contributes to better engine cooling and improved fuel economy.
-
HVAC systems: In heating, ventilation, and air conditioning (HVAC) systems, aluminum is employed in heat exchangers due to its efficient heat transfer capabilities. Its high specific heat capacity enhances the effectiveness of heat exchange processes.
-
Aerospace industry: The lightweight nature and high specific heat capacity of aluminum make it a suitable material for aircraft construction. This contributes to fuel efficiency and improved performance.
Factors Affecting the Specific Heat Capacity of Aluminum
While the value of 900 J/kg·K is a good general approximation, several factors can influence the precise specific heat capacity of aluminum:
-
Purity: Impurities in aluminum can affect its crystalline structure and, consequently, its specific heat capacity. Higher purity aluminum generally exhibits a more consistent specific heat capacity closer to the ideal value.
-
Temperature: The specific heat capacity of aluminum is not entirely constant across all temperature ranges. While the variation is relatively small within typical operating temperatures, it's essential to consider temperature dependency for highly precise calculations. The specific heat capacity generally increases slightly with temperature.
-
Pressure: The influence of pressure on aluminum's specific heat capacity is generally considered negligible for most practical applications. However, at extremely high pressures, the effect may become more pronounced.
-
Alloys: When aluminum is alloyed with other elements to enhance its mechanical properties, the resulting alloy's specific heat capacity might deviate from the value for pure aluminum. The specific heat capacity of the alloy will depend on the composition and properties of the alloying elements.
-
Phase: The specific heat capacity can change depending on the phase of the aluminum. For example, molten aluminum will have a different specific heat capacity compared to solid aluminum.
Measurement of Specific Heat Capacity
Several methods are used to determine the specific heat capacity of aluminum, including:
-
Calorimetry: This classic method involves heating a known mass of aluminum to a specific temperature and then immersing it in a calorimeter containing a known mass of water at a lower temperature. By measuring the temperature change of the water, the specific heat capacity of aluminum can be calculated using the principle of heat exchange.
-
Differential Scanning Calorimetry (DSC): This sophisticated technique precisely measures the heat flow associated with phase transitions and temperature changes in a material. DSC provides accurate data on specific heat capacity as a function of temperature.
-
Thermophysical property measurement systems: These advanced instruments are specifically designed to determine a wide range of thermophysical properties, including specific heat capacity, with high accuracy and precision.
Applications Leveraging Aluminum's Specific Heat Capacity
The importance of aluminum's specific heat capacity is highlighted in a vast array of applications. Let's explore some examples in more detail:
Heat Sinks in Electronics
The performance and longevity of electronic devices are directly impacted by their ability to manage heat effectively. Aluminum's high specific heat capacity makes it ideal for constructing heat sinks, which passively dissipate heat generated by integrated circuits and other components. The heat sink absorbs the heat, then through conduction and convection, transfers the heat to the surrounding environment. This prevents overheating, ensuring reliable and efficient operation of electronic devices.
The design and efficiency of aluminum heat sinks are often optimized based on surface area, fin design, and material properties. A larger surface area allows for improved heat dissipation, and careful fin design can enhance convection. The specific heat capacity is a fundamental factor in determining how much heat the sink can absorb before its temperature significantly increases.
Cookware Design and Functionality
Aluminum's combination of high specific heat capacity and excellent thermal conductivity is essential in the design and functionality of cookware. It distributes heat quickly and evenly across the cooking surface, preventing hot spots and ensuring consistent cooking. This results in more uniform heating of food and better control over the cooking process.
Compared to other materials like stainless steel, aluminum’s high specific heat capacity means it can store more heat energy for a given temperature increase, leading to quicker and more even cooking. This is especially beneficial in applications requiring rapid heating or precise temperature control, such as searing meat or making delicate sauces. The design of cookware often incorporates features like layered materials or induction-compatible bases to further enhance heat distribution and performance.
Automotive Industry: Engine Cooling and Lightweight Design
Aluminum's lightweight and high specific heat capacity properties contribute significantly to fuel efficiency and performance in the automotive industry. It's used extensively in engine blocks, cylinder heads, and other components, reducing vehicle weight and improving fuel economy.
Aluminum’s ability to absorb heat effectively enhances engine cooling systems. The engine generates a substantial amount of heat during operation, and efficient heat dissipation is crucial for maintaining optimal operating temperatures. Aluminum components, along with efficient cooling systems, help to prevent overheating and engine damage. The specific heat capacity contributes to the effectiveness of this heat dissipation process.
Conclusion: The Significance of Aluminum's Specific Heat Capacity
The specific heat capacity of aluminum, approximately 900 J/kg·K, is a crucial material property that underpins its wide range of applications. Its ability to absorb and release significant amounts of heat without drastic temperature changes makes it invaluable in heat sinks for electronics, cookware, automotive components, and numerous other industries. Understanding the factors that influence this property, as well as its accurate measurement, are vital for optimizing aluminum's performance and developing new technologies that leverage its unique thermal characteristics. The ongoing research and development in materials science continue to refine our understanding and expand the applications of this versatile metal.
Latest Posts
Latest Posts
-
Barium Chloride And Sodium Sulphate Reaction
Mar 19, 2025
-
Is A Webcam Input Or Output
Mar 19, 2025
-
What Type Of Bonding Involves The Unequal Sharing Of Electrons
Mar 19, 2025
-
Which Of The Earths Layers Is The Thinnest
Mar 19, 2025
-
Three Particles Are Fixed On An X Axis
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Specific Heat Capacity Of Aluminum . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
