What Is The Si Derived Unit For Density
News Leon
Mar 31, 2025 · 6 min read
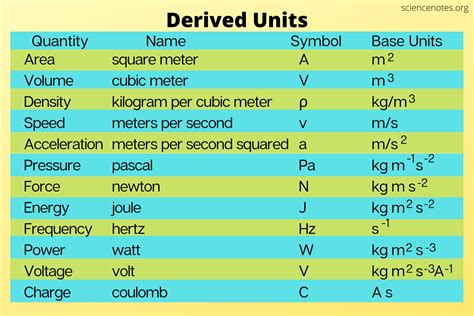
Table of Contents
What is the SI Derived Unit for Density? A Deep Dive into Density and its Measurement
Density, a fundamental concept in physics and chemistry, describes how much mass is packed into a given volume. Understanding density is crucial in numerous fields, from material science and engineering to environmental studies and medicine. But what exactly is the SI derived unit for density, and how is it calculated and used in practical applications? This comprehensive guide will delve into the specifics of density, exploring its definition, units, calculations, and its significance across various disciplines.
Defining Density: Mass per Unit Volume
Density (ρ, pronounced "rho") is defined as the mass of a substance per unit volume. Simply put, it tells us how tightly packed the matter is within a specific space. A substance with high density has a large amount of mass concentrated in a small volume, while a substance with low density has the same mass spread over a larger volume. The mathematical representation of this is:
ρ = m/V
Where:
- ρ represents density
- m represents mass (typically measured in kilograms, kg)
- V represents volume (typically measured in cubic meters, m³)
The SI Derived Unit for Density: Kilograms per Cubic Meter (kg/m³)
The International System of Units (SI) is the globally accepted system for measurement. Since density is derived from mass and volume, its unit is a derived unit, not a base unit. The SI derived unit for density is kilograms per cubic meter (kg/m³). This unit directly reflects the definition of density: mass (kg) divided by volume (m³).
While kg/m³ is the standard SI unit, other units are frequently used in practice, depending on the context and the magnitude of the density being measured. These alternative units are often more convenient for specific applications. We will explore these variations later in the article.
Calculating Density: A Step-by-Step Guide
Calculating the density of a substance involves two key measurements: mass and volume. Here's a step-by-step process:
-
Measure the Mass: Use a balance or scale to accurately determine the mass (m) of the substance in kilograms (kg). Ensure the instrument is calibrated correctly for accurate results.
-
Measure the Volume: The method for measuring volume depends on the state of the substance:
-
Solids with regular shapes (e.g., cubes, cylinders): Use geometric formulas to calculate volume. For example, the volume of a cube is side³, and the volume of a cylinder is πr²h (where r is the radius and h is the height).
-
Solids with irregular shapes: Use water displacement method. Submerge the solid in a graduated cylinder filled with a known volume of water. The difference between the initial and final water levels represents the volume of the solid.
-
Liquids: Use a graduated cylinder or other volumetric glassware to directly measure the volume.
-
Gases: The volume of a gas is dependent on pressure and temperature and requires more complex calculations involving the ideal gas law (PV=nRT).
-
-
Calculate Density: Once you have the mass (m) and volume (V), use the formula ρ = m/V to calculate the density.
Density in Different States of Matter: Solids, Liquids, and Gases
Density varies significantly depending on the state of matter. Generally:
-
Solids: Solids tend to have the highest densities because their particles are tightly packed together. Exceptions exist, of course, depending on the atomic structure and bonding.
-
Liquids: Liquids have intermediate densities, as their particles are closer together than in gases but not as tightly packed as in solids.
-
Gases: Gases have the lowest densities because their particles are widely dispersed and have large spaces between them. The density of gases is highly sensitive to changes in temperature and pressure.
Practical Applications of Density: Across Diverse Fields
The concept of density is crucial in various fields. Here are a few examples:
-
Material Science and Engineering: Density is a key factor in material selection for various applications. Engineers consider density when designing structures, vehicles, and other products to optimize weight and strength. For example, aerospace engineers prioritize materials with low density to reduce fuel consumption.
-
Environmental Science: Density plays a critical role in understanding environmental processes. Water density variations, for instance, drive ocean currents and affect marine life. Soil density influences plant growth and nutrient availability.
-
Medicine: Body density measurements are used to assess body composition and health status. For example, bone density measurements help diagnose osteoporosis. Density gradients are also used in various laboratory techniques for separating biological samples.
-
Geology: Rock density is essential for geophysical studies and mineral exploration. Density differences help geologists identify different rock formations and locate subsurface resources.
-
Food Science: Density is a critical factor in food processing and quality control. For example, the density of milk is an indicator of its fat content.
-
Meteorology: Air density is a key factor influencing weather patterns and atmospheric circulation. Changes in air density due to temperature and pressure differences drive wind and other weather phenomena.
Alternative Units of Density: Beyond kg/m³
While kg/m³ is the standard SI unit, various other units are commonly used, particularly in specific contexts:
-
g/cm³: Grams per cubic centimeter is a frequently used unit, especially in chemistry and material science. It's easily convertible to kg/m³ (1 g/cm³ = 1000 kg/m³).
-
kg/L: Kilograms per liter is another commonly used unit, particularly when dealing with liquids. It's directly related to kg/m³ (1 kg/L = 1000 kg/m³).
-
lb/ft³: Pounds per cubic foot is commonly used in engineering applications in countries using the imperial system.
-
Specific Gravity: Specific gravity is the ratio of a substance's density to the density of water at a specified temperature (usually 4°C). It's a dimensionless quantity often used to compare the relative densities of different materials.
Density and Temperature: The Effect of Thermal Expansion
Temperature significantly impacts density. As temperature increases, most substances expand, resulting in an increase in volume and a decrease in density. This is known as thermal expansion. Conversely, as temperature decreases, most substances contract, leading to a decrease in volume and an increase in density. Water is a notable exception to this rule; it exhibits unusual density behavior around its freezing point.
Density and Pressure: Compressibility of Matter
Pressure also affects density, particularly in gases and liquids. Increasing pressure compresses a substance, reducing its volume and increasing its density. Solids are generally less compressible than liquids and gases, exhibiting a smaller change in density with pressure changes.
Conclusion: Density – A Cornerstone of Scientific Understanding
Density, a seemingly simple concept, is a cornerstone of scientific understanding across diverse fields. Its precise measurement and understanding are crucial for various applications, from designing efficient structures to monitoring environmental changes and advancing medical technologies. While the SI derived unit for density is kg/m³, the choice of unit often depends on the specific application and the magnitude of the density being measured. Understanding the relationships between density, mass, volume, temperature, and pressure is fundamental to comprehending the physical properties of matter and its behavior in various systems. This comprehensive understanding allows scientists and engineers to make informed decisions and develop innovative solutions in numerous disciplines.
Latest Posts
Latest Posts
-
Pb Oh 2 Hcl H2o Pbcl2
Apr 02, 2025
-
Is Ethanol More Polar Than Water
Apr 02, 2025
-
Cis 1 Tert Butyl 2 Methylcyclohexane
Apr 02, 2025
-
Whats The Difference Between Weathering And Erosion
Apr 02, 2025
-
Evaluate The Geometric Series Or State That It Diverges
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Si Derived Unit For Density . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
