What Is The Shape Of Ch3
News Leon
Mar 30, 2025 · 5 min read
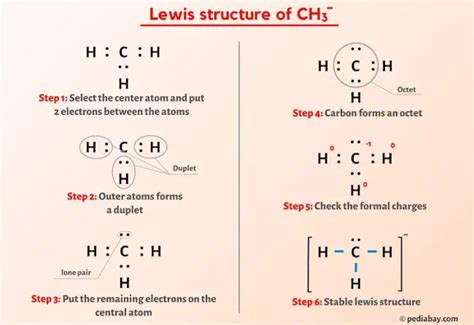
Table of Contents
What is the Shape of CH3? Understanding Molecular Geometry
The question, "What is the shape of CH3?" might seem deceptively simple, but delving into it reveals fascinating insights into the world of molecular geometry and the behavior of molecules. The answer isn't a simple single shape, but rather depends on the context – specifically, whether we're talking about the methyl radical (CH3•) or the methyl cation (CH3+) or methyl anion (CH3-). Let's explore each in detail.
Understanding Molecular Geometry: VSEPR Theory
Before diving into the shapes of CH3 species, it's crucial to understand the fundamental principles governing molecular geometry. The Valence Shell Electron Pair Repulsion (VSEPR) theory is the cornerstone of this understanding. VSEPR theory posits that electron pairs – both bonding and non-bonding (lone pairs) – around a central atom repel each other, arranging themselves to minimize this repulsion. This arrangement dictates the overall shape of the molecule. The key is to consider both bonding and non-bonding electron pairs.
Key Concepts in VSEPR Theory:
- Electron Domains: These include both bonding pairs (electrons shared between atoms) and lone pairs (electrons not involved in bonding).
- Electron Domain Geometry: This describes the arrangement of electron domains around the central atom.
- Molecular Geometry: This describes the arrangement of only the atoms in the molecule. Lone pairs influence the molecular geometry, but are not included in its description.
The Methyl Radical (CH3•): A Trigonal Planar Geometry?
The methyl radical (CH3•) possesses a single unpaired electron on the carbon atom. While VSEPR theory typically focuses on electron pairs, the presence of a single electron still influences the overall arrangement. Applying VSEPR to CH3•, we consider three bonding electron pairs and one unpaired electron. While this might lead you to believe a tetrahedral arrangement, the unpaired electron occupies a p-orbital, resulting in a trigonal planar geometry for the CH3• radical. This arrangement minimizes electron-electron repulsion.
Why not Tetrahedral?
A tetrahedral geometry would seem logical at first glance, given the four electron domains. However, the unpaired electron behaves differently than a lone pair. A lone pair occupies more space than a bonding pair, leading to greater repulsion. An unpaired electron doesn't exert the same strong repulsive force. The result is a flatter, trigonal planar structure.
Experimental Evidence:
Experimental data, such as spectroscopic studies (e.g., electron spin resonance, ESR), supports the trigonal planar geometry of the methyl radical. The observed bond angles and spectral signatures are consistent with this structure.
The Methyl Cation (CH3+): A Trigonal Planar Structure
The methyl cation (CH3+) has only three bonding electron pairs surrounding the central carbon atom. According to VSEPR theory, with three bonding pairs and zero lone pairs, the electron domain geometry and the molecular geometry are both trigonal planar. The carbon atom is sp<sup>2</sup> hybridized, which further supports this planar arrangement. The three C-H bonds are arranged symmetrically around the carbon atom, with bond angles of approximately 120°.
Stability Considerations:
The methyl cation is highly reactive due to its positive charge and incomplete octet on the carbon atom. This instability contributes to its participation in many chemical reactions, making it an important intermediate in various processes.
The Methyl Anion (CH3-): A Trigonal Pyramidal Geometry?
The methyl anion (CH3-) presents a slightly more complex scenario. Here, we have three bonding electron pairs and one lone pair around the central carbon atom. This gives a tetrahedral electron domain geometry. However, the molecular geometry, considering only the atoms, is trigonal pyramidal. The lone pair occupies more space than the bonding pairs, pushing the three hydrogen atoms closer together, resulting in a pyramidal shape.
Angle Deviation:
The bond angles in CH3- deviate from the ideal tetrahedral angle (109.5°) and are slightly less. The lone pair's influence creates a compression in the H-C-H bond angles. The precise angle depends on the level of theory used in computational studies.
Reactivity and Stability:
Similar to the methyl cation, the methyl anion is also quite reactive due to its negative charge and the availability of the lone pair to participate in bonding. It tends to act as a nucleophile in chemical reactions.
Computational Chemistry and Modeling
Computational methods, such as density functional theory (DFT) and Hartree-Fock calculations, play a crucial role in determining molecular geometries, particularly for unstable species like radicals and ions. These methods can accurately predict bond lengths, bond angles, and overall shapes, corroborating experimental observations or providing insights where experimental data are limited.
Conclusion: Context is Key
The shape of "CH3" is not a single, definitive answer. The geometry depends entirely on the charge and the presence of any unpaired electrons. The methyl radical (CH3•) exhibits a trigonal planar geometry, the methyl cation (CH3+) has a trigonal planar shape, and the methyl anion (CH3-) displays a trigonal pyramidal shape. Understanding VSEPR theory and employing computational methods are crucial tools to predict and explain these different molecular geometries. Each species displays unique reactivity influenced by its specific molecular structure, underscoring the importance of considering the electronic configuration when discussing the shape and behavior of molecules.
Further Exploration:
- Hybridization: Understanding the concept of orbital hybridization (sp<sup>2</sup>, sp<sup>3</sup>) is key to fully grasping the bonding and geometry of these species.
- Advanced Computational Chemistry: Delving deeper into computational methods provides a more quantitative understanding of bond lengths, bond angles, and energy levels.
- Spectroscopic Techniques: Exploring different spectroscopic techniques (NMR, IR, ESR) offers experimental methods to confirm the predicted geometries.
By delving into the specifics of each CH3 species, we highlight the importance of considering the entire electronic environment when determining the shape of a molecule. The subtle differences in electronic configuration lead to significant variations in geometry and reactivity. This deeper understanding is crucial for predicting and explaining chemical behavior.
Latest Posts
Latest Posts
-
Why Electronic Configuration Of Calcium Is 2 8 8 2
Apr 01, 2025
-
1 Square Meter Is How Many Square Centimeters
Apr 01, 2025
-
Distance Between A Line And Plane
Apr 01, 2025
-
Are All Cells The Same Shape And Size
Apr 01, 2025
-
Determine The Number Of 3 D Electrons In Cr
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Shape Of Ch3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
