Why Electronic Configuration Of Calcium Is 2 8 8 2
News Leon
Apr 01, 2025 · 6 min read
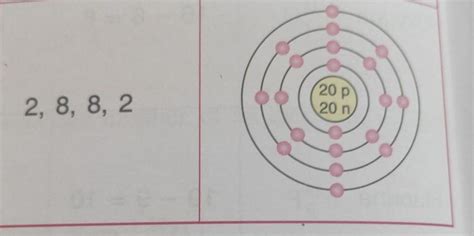
Table of Contents
Why is the Electronic Configuration of Calcium 2, 8, 8, 2? A Deep Dive into Atomic Structure
The seemingly simple electronic configuration of calcium, 2, 8, 8, 2, belies a fascinating story about the fundamental building blocks of matter and the rules that govern their arrangement. Understanding this configuration requires delving into the intricacies of atomic structure, quantum mechanics, and the principles that dictate electron placement within an atom. This article will provide a comprehensive explanation, addressing common misconceptions and exploring the underlying physics.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we tackle calcium's electronic configuration, let's establish a foundational understanding of atomic structure. An atom consists of three fundamental particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's atomic number and its identity. Calcium (Ca) has an atomic number of 20, meaning it possesses 20 protons.
- Neutrons: Neutrally charged particles also found within the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. The number of electrons is typically equal to the number of protons in a neutral atom. It's the arrangement of these electrons that determines the atom's chemical properties and reactivity.
Electron Shells and Subshells: The Quantum Mechanical Model
Electrons don't orbit the nucleus randomly. Their behavior is governed by the principles of quantum mechanics, which dictate that electrons occupy specific energy levels, often visualized as shells. These shells are further divided into subshells, which are designated by letters (s, p, d, f), each with a specific number of orbitals. Orbitals are regions of space where there's a high probability of finding an electron.
- Shell 1 (n=1): Contains only one subshell, the 1s subshell, which can hold a maximum of two electrons.
- Shell 2 (n=2): Contains two subshells: the 2s (holding up to two electrons) and the 2p (holding up to six electrons).
- Shell 3 (n=3): Contains three subshells: 3s (two electrons), 3p (six electrons), and 3d (ten electrons).
- Shell 4 (n=4): Contains four subshells: 4s (two electrons), 4p (six electrons), 4d (ten electrons), and 4f (fourteen electrons). And so on...
The Aufbau Principle and Hund's Rule: Filling the Shells
The arrangement of electrons within these shells and subshells follows specific rules:
- The Aufbau Principle (Building-Up Principle): Electrons fill the lowest energy levels first. This means that the 1s subshell fills before the 2s, the 2s before the 2p, and so on. The order of filling is determined by the increasing energy levels of the subshells.
- Hund's Rule: Within a subshell, electrons fill each orbital individually before pairing up. This minimizes electron-electron repulsion and results in a more stable configuration.
Deriving Calcium's Electronic Configuration (2, 8, 8, 2)
Now, let's apply these principles to calcium (atomic number 20):
- Shell 1 (n=1): The 1s subshell can hold two electrons. Calcium's first two electrons fill this shell completely: 1s².
- Shell 2 (n=2): The 2s subshell holds two electrons (2s²), and the 2p subshell can hold six (2p⁶). Together, shell 2 is filled with eight electrons.
- Shell 3 (n=3): The 3s subshell holds two electrons (3s²), and the 3p subshell holds six (3p⁶). This shell also accommodates eight electrons.
- Shell 4 (n=4): After filling the lower energy levels, the remaining two electrons occupy the 4s subshell (4s²).
Therefore, the complete electronic configuration of calcium is 1s²2s²2p⁶3s²3p⁶4s². This is often simplified and represented as 2, 8, 8, 2, indicating the number of electrons in each principal energy level.
Why Not a Different Configuration? The Stability of Filled Subshells
You might wonder why calcium's electrons don't arrange themselves differently. The answer lies in the stability associated with filled subshells. Filled subshells and completely filled outer shells represent a state of low energy and high stability. Calcium's configuration, with filled 1s, 2s, 2p, 3s, and 3p subshells, and a filled 4s subshell, is energetically favorable and significantly more stable than any alternative configuration. Attempting to force electrons into higher energy levels without filling the lower ones would require a substantial input of energy.
Exceptions to the Aufbau Principle: The Subtleties of Electron Configuration
While the Aufbau principle provides a generally reliable framework for predicting electron configurations, there are exceptions, particularly in transition metals and lanthanides/actinides. These exceptions arise due to the subtle energy differences between subshells and the influence of electron-electron interactions. The relative energies of the 3d and 4s subshells, for instance, are quite close, and sometimes it's energetically more favorable for electrons to occupy the 3d subshell before completely filling the 4s. These exceptions are explained by more complex quantum mechanical calculations that go beyond the scope of this introductory discussion. However, it is important to note that Calcium does not exhibit such an exception. Its configuration precisely follows the Aufbau principle.
The Significance of Calcium's Electronic Configuration in its Chemistry
Calcium's electronic configuration directly dictates its chemical behavior. The two electrons in the outermost 4s subshell are relatively loosely bound to the nucleus. This makes calcium highly reactive, readily losing these two electrons to form a stable 2+ ion (Ca²⁺). This tendency to lose electrons explains calcium's properties:
- Reactivity: Calcium readily reacts with water, acids, and oxygen, often releasing hydrogen gas or heat in the process.
- Ionic Bonding: Calcium readily forms ionic compounds by electrostatic attraction with negatively charged ions, like chloride (Cl⁻) to form calcium chloride (CaCl₂).
- Metallic Bonding: Calcium exhibits metallic bonding, contributing to its properties as a silvery-white metal that is relatively soft and malleable.
Common Misconceptions about Electronic Configuration
Several common misconceptions surround electronic configurations:
- The Bohr Model Oversimplification: While the Bohr model is useful for a basic understanding, it's inaccurate to depict electrons as orbiting the nucleus in neat, circular paths. Quantum mechanics provides a more accurate, albeit more complex, representation.
- Electron Configuration as a Static Picture: The electronic configuration represents the most probable distribution of electrons, not a fixed, static arrangement. Electrons are constantly moving and their positions are probabilistic.
- Ignoring Electron-Electron Interactions: Simplified explanations often overlook the significant influence of electron-electron repulsion on electron configuration.
Conclusion: A Fundamental Building Block
The seemingly simple electronic configuration of calcium (2, 8, 8, 2) is a consequence of fundamental principles governing atomic structure and quantum mechanics. Understanding this configuration is essential for comprehending calcium's chemical properties and its role in various chemical reactions and biological processes. While exceptions exist, calcium's straightforward electron arrangement serves as a clear and illustrative example of the Aufbau principle and the stability associated with filled subshells, reinforcing the fundamental concepts of atomic theory. The detailed explanation provided above clarifies the underlying physics and addresses common misconceptions, providing a robust foundation for further exploration of atomic and chemical principles. By understanding this seemingly simple configuration, we gain a deeper appreciation for the complexity and elegance of the natural world at its most fundamental level.
Latest Posts
Latest Posts
-
How Many Grams Are In 5 66 Mol Of Caco3
Apr 02, 2025
-
What Is 13 25 As A Decimal
Apr 02, 2025
-
How Many Months Are In Five Years
Apr 02, 2025
-
Geometric Mean Of 9 And 4
Apr 02, 2025
-
A Codon Consists Of How Many Bases
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Why Electronic Configuration Of Calcium Is 2 8 8 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
