Determine The Number Of 3 D Electrons In Cr .
News Leon
Apr 01, 2025 · 4 min read
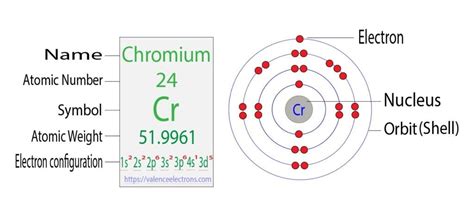
Table of Contents
Determining the Number of 3d Electrons in Chromium: A Deep Dive
Chromium (Cr), a transition metal with atomic number 24, presents a fascinating case study in electron configuration and the exceptions to Hund's rule. Understanding its electronic structure, particularly the number of 3d electrons, requires a deep dive into atomic orbitals, electron filling rules, and the nuances of electronic stability. This article will comprehensively explore this topic, providing a clear and detailed explanation for both beginners and those seeking a more advanced understanding.
Electron Configuration Fundamentals
Before delving into chromium's specific case, let's review the fundamental principles governing electron configuration. The arrangement of electrons in an atom is described by its electronic configuration, which dictates its chemical properties and reactivity. This configuration follows specific rules:
Aufbau Principle:
This principle dictates that electrons fill atomic orbitals starting from the lowest energy level and progressing upwards. The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Pauli Exclusion Principle:
No two electrons in an atom can have the same set of four quantum numbers (n, l, ml, ms). This means each orbital can hold a maximum of two electrons with opposite spins (+1/2 and -1/2).
Hund's Rule:
When filling orbitals of equal energy (degenerate orbitals, such as the five 3d orbitals), electrons initially occupy each orbital singly with parallel spins before pairing up. This maximizes the total spin and minimizes electron-electron repulsion.
The Chromium Anomaly: Half-Filled Stability
The expected electron configuration for chromium based on the Aufbau principle would be 1s²2s²2p⁶3s²3p⁶4s²3d⁴. However, the actual configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d⁵. This deviation is a crucial point and is due to the exceptional stability associated with half-filled and completely filled d orbitals.
Why the Exception?
A half-filled d subshell (d⁵) offers enhanced stability compared to a partially filled d subshell (d⁴). This stability arises from several factors:
-
Exchange Energy: Electrons with parallel spins in different orbitals experience a stabilizing exchange interaction. A half-filled d subshell maximizes this exchange energy, significantly lowering the overall energy of the atom.
-
Symmetrical Electron Distribution: A half-filled d shell leads to a more symmetrical distribution of electron density, reducing electron-electron repulsion.
-
Increased Ionization Energy: Atoms with half-filled or fully filled d-subshells possess higher ionization energies, making them less reactive.
By promoting one electron from the 4s orbital to the 3d orbital, chromium achieves this highly stable half-filled 3d⁵ configuration. The energy gained from the increased exchange energy and reduced electron-electron repulsion outweighs the energy cost of promoting the electron.
Determining the Number of 3d Electrons in Chromium
Given the actual electron configuration of chromium (1s²2s²2p⁶3s²3p⁶4s¹3d⁵), we can definitively state that chromium has five 3d electrons. The presence of a single electron in the 4s orbital is crucial in enabling this energetically favorable configuration.
Illustrative Diagrams and Orbital Filling
Visualizing the electron configuration can aid understanding. Consider the following:
Incorrect (based solely on Aufbau Principle):
4s: ↑↓
3d: ↑ ↑ ↑ ↑
Correct (reflecting half-filled stability):
4s: ↑
3d: ↑ ↑ ↑ ↑ ↑
Each arrow represents an electron, and the direction indicates the electron spin. The correct diagram shows five electrons individually occupying the five 3d orbitals, showcasing the half-filled stability.
Implications of the 3d⁵ Configuration
The five 3d electrons in chromium significantly influence its properties:
-
Magnetic Properties: The unpaired electrons in the 3d orbitals make chromium paramagnetic, meaning it is attracted to external magnetic fields.
-
Oxidation States: Chromium exhibits multiple oxidation states, notably +2, +3, and +6. These different oxidation states arise from the variable number of electrons that can be lost from the 4s and 3d orbitals.
-
Chemical Reactivity: While chromium is relatively unreactive compared to other transition metals, its capacity to form various oxidation states leads to diverse chemical behavior and compound formation.
Comparison with Other Transition Metals
It's instructive to compare chromium's electron configuration with other transition metals. Many transition metals exhibit similar deviations from the Aufbau principle to achieve half-filled or fully filled d subshells, illustrating the importance of these stable configurations.
Advanced Considerations: Relativistic Effects
At higher atomic numbers, relativistic effects begin to play a more significant role in electron configuration. These effects subtly influence the energy levels of electrons and can affect the order of filling. While not a major factor in chromium's configuration, it's essential to acknowledge their growing importance in heavier elements.
Conclusion: The Significance of Half-Filled Stability
Determining the number of 3d electrons in chromium highlights the importance of understanding the interplay between the Aufbau principle, Hund's rule, and the exceptional stability associated with half-filled and fully filled d orbitals. The deviation from the strictly predicted electron configuration showcases the complex and dynamic nature of electron arrangement within atoms and provides a foundation for understanding the diverse properties of transition metals. The five 3d electrons in chromium are not merely a numerical detail; they are fundamental to its unique chemical and physical characteristics. This knowledge forms a critical foundation for comprehending the behavior of chromium in various chemical and physical scenarios, including its applications in diverse fields such as metallurgy, catalysis, and pigment production.
Latest Posts
Latest Posts
-
How Many Grams Are In 5 66 Mol Of Caco3
Apr 02, 2025
-
What Is 13 25 As A Decimal
Apr 02, 2025
-
How Many Months Are In Five Years
Apr 02, 2025
-
Geometric Mean Of 9 And 4
Apr 02, 2025
-
A Codon Consists Of How Many Bases
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Determine The Number Of 3 D Electrons In Cr . . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
