What Is The Reagent Required To Accomplish The Following Transformation
News Leon
Mar 28, 2025 · 6 min read
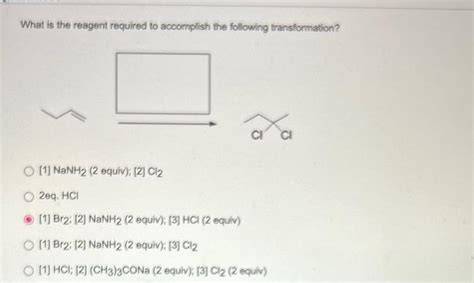
Table of Contents
What Reagent is Required to Accomplish the Following Transformation? A Comprehensive Guide
Organic chemistry transformations are the backbone of synthetic chemistry, allowing us to build complex molecules from simpler precursors. Understanding which reagents are required for specific transformations is crucial for any chemist, whether a seasoned professional or a budding student. This article will delve into the principles behind reagent selection for various organic transformations, providing a detailed explanation with examples. We'll explore several common reaction types and discuss the key considerations when choosing the appropriate reagent.
Understanding Reaction Mechanisms: The Key to Reagent Selection
The most important factor in choosing the right reagent is understanding the underlying reaction mechanism. Different reagents induce different mechanistic pathways, leading to different products. Knowing the desired transformation and the mechanism it requires allows for the accurate selection of reagents. Let's look at some key mechanisms:
1. Nucleophilic Substitution Reactions (SN1 & SN2)
These reactions involve the substitution of one nucleophile for another. The choice of reagent hinges on the substrate's structure and the reaction conditions.
-
SN2 Reactions: These reactions proceed via a concerted mechanism, meaning the bond breaking and bond formation occur simultaneously. Strong nucleophiles are required, and steric hindrance around the electrophilic carbon significantly affects the reaction rate. Common reagents include:
- Sodium iodide (NaI) in acetone: Excellent for primary and secondary alkyl halides.
- Potassium hydroxide (KOH) in ethanol: A versatile base and nucleophile.
- Sodium cyanide (NaCN): Introduces a nitrile group.
- Sodium azide (NaN3): Introduces an azide group.
-
SN1 Reactions: These reactions proceed via a two-step mechanism involving the formation of a carbocation intermediate. Weak nucleophiles can be used, and tertiary alkyl halides react faster due to greater carbocation stability. Common reagents include:
- Water (H2O): A weak nucleophile that can participate in SN1 reactions.
- Alcohols (ROH): Can act as both nucleophiles and solvents.
- Silver nitrate (AgNO3): Promotes ionization of alkyl halides, facilitating SN1 reactions.
2. Elimination Reactions (E1 & E2)
Elimination reactions involve the removal of atoms or groups from a molecule, typically resulting in the formation of a double bond (alkene).
-
E2 Reactions: These reactions are concerted, requiring a strong base to abstract a proton and simultaneous elimination of a leaving group. The stereochemistry of the starting material is crucial. Common reagents include:
- Potassium tert-butoxide (t-BuOK): A strong, bulky base favoring the formation of less substituted alkenes (Hofmann product).
- Sodium ethoxide (NaOEt): A strong base often used in elimination reactions.
- Potassium hydroxide (KOH): Can act as a base in elimination reactions.
-
E1 Reactions: These reactions proceed via a two-step mechanism, involving the formation of a carbocation intermediate followed by the loss of a proton. They typically require relatively acidic conditions. Common reagents include:
- Strong acids (e.g., H2SO4, H3PO4): Promote the formation of carbocations.
- Heat: Often required to facilitate the reaction.
3. Addition Reactions
Addition reactions involve the addition of atoms or groups to a molecule, typically across a double or triple bond.
-
Electrophilic Addition: These reactions involve the addition of an electrophile to a double or triple bond. Common reagents include:
- Halogens (Cl2, Br2, I2): Add across the double bond.
- Hydrogen halides (HCl, HBr, HI): Add across the double bond, following Markovnikov's rule.
- Water (H2O) with an acid catalyst (H2SO4): Adds across the double bond to form an alcohol (hydration).
-
Nucleophilic Addition: These reactions involve the addition of a nucleophile to a carbonyl group (C=O). Common reagents include:
- Grignard reagents (RMgX): Add to carbonyl groups to form alcohols.
- Organolithium reagents (RLi): Similar to Grignard reagents.
- Hydride reducing agents (LiAlH4, NaBH4): Reduce carbonyl groups to alcohols.
4. Oxidation and Reduction Reactions
These reactions involve the change in oxidation state of a molecule.
-
Oxidation: Increase in oxidation state. Common reagents include:
- Potassium permanganate (KMnO4): A strong oxidizing agent.
- Potassium dichromate (K2Cr2O7): A strong oxidizing agent.
- Jones reagent (CrO3 in H2SO4): Oxidizes primary alcohols to carboxylic acids and secondary alcohols to ketones.
-
Reduction: Decrease in oxidation state. Common reagents include:
- Lithium aluminum hydride (LiAlH4): A powerful reducing agent.
- Sodium borohydride (NaBH4): A milder reducing agent.
- Hydrogen gas (H2) with a metal catalyst (Pd, Pt, Ni): Catalytic hydrogenation.
Practical Considerations in Reagent Selection
Beyond the reaction mechanism, several other factors influence reagent choice:
-
Selectivity: The ability of a reagent to react preferentially with one functional group over another. A highly selective reagent is crucial when multiple reactive groups are present.
-
Yield: The amount of product obtained relative to the starting material. Higher yields are always desirable.
-
Cost: The price of the reagent and its availability can significantly impact the choice.
-
Toxicity and Safety: The toxicity and environmental impact of the reagents are important considerations. Safer and greener alternatives are often preferred.
-
Reaction Conditions: Temperature, pressure, and solvent can greatly affect the reaction outcome and need to be carefully considered in conjunction with reagent selection.
Case Studies: Illustrative Examples
Let's examine specific transformations and the reagents needed:
1. Conversion of an Alkyl Halide to an Alcohol:
- Starting material: 1-bromobutane
- Desired product: 1-butanol
- Reaction type: Nucleophilic substitution (SN2)
- Reagent: Sodium hydroxide (NaOH) in water. NaOH acts as a nucleophile, replacing the bromide ion with a hydroxide ion.
2. Conversion of an Alcohol to an Alkene:
- Starting material: 2-butanol
- Desired product: 2-butene
- Reaction type: Elimination (E1 or E2)
- Reagent: Concentrated sulfuric acid (H2SO4) and heat. Acid promotes dehydration of the alcohol, leading to alkene formation.
3. Conversion of an Alkene to an Alcohol:
- Starting material: Ethene
- Desired product: Ethanol
- Reaction type: Electrophilic addition
- Reagent: Water (H2O) with an acid catalyst (H2SO4). This promotes hydration of the alkene, leading to alcohol formation.
4. Conversion of a Ketone to a Secondary Alcohol:
- Starting material: Propanone
- Desired product: Propan-2-ol
- Reaction type: Reduction
- Reagent: Sodium borohydride (NaBH4). NaBH4 is a mild reducing agent that reduces ketones to secondary alcohols.
5. Conversion of a Carboxylic Acid to an Alcohol:
- Starting material: Ethanoic acid
- Desired product: Ethanol
- Reaction type: Reduction
- Reagent: Lithium aluminum hydride (LiAlH4). LiAlH4 is a powerful reducing agent capable of reducing carboxylic acids to alcohols.
Conclusion
Choosing the appropriate reagent for a specific organic transformation is a multifaceted process requiring a deep understanding of reaction mechanisms, selectivity, yield, cost, and safety. This article has explored several common reaction types and the reagents typically employed. However, this is not an exhaustive list, and further research is always recommended to optimize reaction conditions and choose the most suitable reagent for a given transformation. The principles outlined here provide a strong foundation for approaching reagent selection systematically, paving the way for successful synthesis in organic chemistry. Careful consideration of all factors will lead to efficient and effective organic synthesis. Remember that practice and familiarity with reaction mechanisms are key to mastering reagent selection.
Latest Posts
Latest Posts
-
Which Elements Have 5 Valence Electrons
Mar 31, 2025
-
Which Of The Following Is An Example Of Nonvolatile Memory
Mar 31, 2025
-
How To Calculate Voltage Of A Cell
Mar 31, 2025
-
Explain The Importance Of Ph Testing In Everyday Life
Mar 31, 2025
-
Sublimation Is Physical Or Chemical Change
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Reagent Required To Accomplish The Following Transformation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
