Sublimation Is Physical Or Chemical Change
News Leon
Mar 31, 2025 · 5 min read
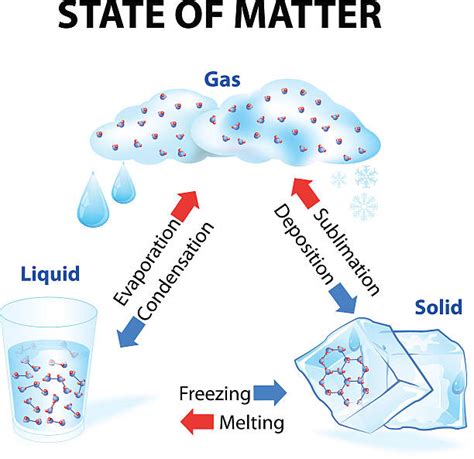
Table of Contents
Is Sublimation a Physical or Chemical Change? A Deep Dive
The question of whether sublimation is a physical or chemical change often sparks debate. Understanding this requires a firm grasp of the fundamental differences between physical and chemical changes. This comprehensive guide delves into the intricacies of sublimation, exploring its nature and definitively answering the central question. We'll also explore related concepts and practical applications to provide a holistic understanding.
Understanding Physical and Chemical Changes
Before we tackle sublimation, let's establish a clear understanding of the distinction between physical and chemical changes.
Physical changes alter the form or appearance of a substance but do not change its chemical composition. Think of cutting paper, melting ice, or dissolving sugar in water. The substance remains the same; only its physical state or form is altered. These changes are often reversible.
Chemical changes, also known as chemical reactions, result in the formation of new substances with different chemical properties. Burning wood, rusting iron, and baking a cake are all examples. The original substances are transformed into entirely new ones, a process often irreversible. These changes are usually accompanied by observable signs like a change in color, temperature, or the production of gas.
Sublimation: A Definition
Sublimation is a phase transition where a substance transitions directly from a solid to a gas without passing through the intermediate liquid phase. The reverse process, where a gas transitions directly to a solid, is called deposition.
Think of dry ice (solid carbon dioxide). At room temperature and atmospheric pressure, it doesn't melt into a liquid; instead, it directly sublimes into carbon dioxide gas. Similarly, snow and ice can sublime on a cold, windy day.
Is Sublimation a Physical or Chemical Change? The Answer
Sublimation is a physical change. While the state of matter changes dramatically from solid to gas, the chemical composition of the substance remains unchanged. No new substances are formed during sublimation. The molecules of the substance simply gain enough kinetic energy to overcome the intermolecular forces holding them in a solid structure, transitioning into the gaseous phase. The reverse process, deposition, further reinforces this: the same molecules simply lose kinetic energy and return to a solid state. No chemical bonds are broken or formed throughout the entire process.
Evidence Supporting Sublimation as a Physical Change
Several key observations support the classification of sublimation as a physical change:
- No new substances are formed: The chemical formula of the substance before and after sublimation remains identical. For example, dry ice (CO₂) sublimes into carbon dioxide gas (CO₂). The chemical composition remains unchanged.
- Reversibility: Sublimation is a reversible process. The gas produced through sublimation can undergo deposition to return to its solid form, demonstrating the preservation of the original substance.
- Changes in physical properties only: While the physical state changes (from solid to gas), the intrinsic properties of the substance, such as its chemical formula and molar mass, remain consistent.
- Energy changes are physical in nature: The energy required for sublimation is used to overcome intermolecular forces, not to break chemical bonds. This energy change is consistent with physical changes involving alterations in kinetic energy and state of matter.
Common Examples of Sublimation
Understanding sublimation is easier with real-world examples:
- Dry ice: As mentioned, dry ice (solid CO₂) sublimes directly into gaseous CO₂ at room temperature.
- Naphthalenes (mothballs): These gradually disappear over time due to sublimation, leaving no liquid residue.
- Frost formation: Water vapor in the air can directly deposit onto cold surfaces as ice crystals, demonstrating deposition, the reverse of sublimation.
- Freeze-drying: This preservation technique utilizes sublimation to remove water from food products, preserving their quality.
- Iodine crystals: When heated gently, iodine crystals sublime, producing a characteristic purple vapor that can then deposit as crystals upon cooling.
Sublimation vs. Evaporation and Boiling
It's crucial to differentiate sublimation from evaporation and boiling, which are also phase transitions:
- Evaporation: This involves a liquid transitioning to a gas. The process is slower than boiling and occurs only at the surface of the liquid.
- Boiling: This involves a liquid transitioning to a gas at its boiling point, forming bubbles throughout the liquid.
Sublimation differs fundamentally because it involves a direct solid-to-gas transition, bypassing the liquid phase entirely. This makes it a unique and interesting physical process.
Factors Affecting Sublimation Rate
Several factors influence the rate at which sublimation occurs:
- Temperature: Higher temperatures generally accelerate sublimation.
- Pressure: Lower pressures favor sublimation. This explains why dry ice sublimes readily at atmospheric pressure.
- Surface area: A larger surface area allows for more molecules to escape into the gaseous phase, increasing the sublimation rate.
- Nature of the substance: Some substances sublime more readily than others due to their inherent intermolecular forces.
Applications of Sublimation
Sublimation finds diverse applications in various fields:
- Purification of substances: Sublimation can purify substances by separating volatile components from less volatile ones.
- Printing and Dyeing: Sublimation printing is used to create vibrant, long-lasting images on fabrics and other materials.
- Microscopy: Sublimation is used in techniques to prepare samples for electron microscopy.
- Food preservation: Freeze-drying utilizes sublimation to remove water from food, preserving its quality and extending shelf life.
- Forensic science: Sublimation can be used to analyze various substances in forensic investigations.
Conclusion: Sublimation - A Definitive Physical Change
In conclusion, sublimation is undeniably a physical change. It involves a change in the physical state of a substance, from solid to gas, without any alteration in its chemical composition. The reversible nature of the process, the absence of new substance formation, and the fact that only intermolecular forces are overcome, all strongly support its classification as a physical change. While seemingly dramatic, the transition involves only changes in the energy and arrangement of molecules, not their fundamental structure. Understanding this distinction is key to grasping the fundamentals of matter and its phase transitions. The wide-ranging applications of sublimation further highlight its importance in various scientific and industrial fields.
Latest Posts
Latest Posts
-
Determine The Area Of The Shaded Region In The Figure
Apr 01, 2025
-
A Tt Pea Plant Is A
Apr 01, 2025
-
Common Factors Of 8 And 36
Apr 01, 2025
-
The Needle On A Compass Always Points Towards What Direction
Apr 01, 2025
-
How Many Ounces In 1 8 Pound
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Sublimation Is Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
