Explain The Importance Of Ph Testing In Everyday Life
News Leon
Mar 31, 2025 · 6 min read
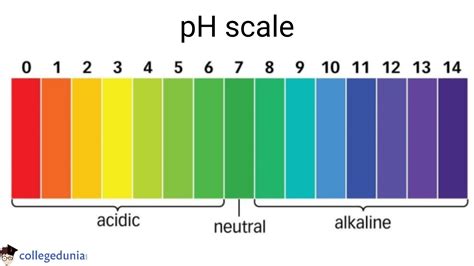
Table of Contents
The Unsung Hero of Everyday Life: Understanding the Importance of pH Testing
pH testing, the measurement of how acidic or alkaline a substance is, might sound like a niche scientific process. However, its importance extends far beyond the laboratory, significantly impacting various aspects of our daily lives. From the food we consume to the health of our bodies and the environment around us, understanding and utilizing pH testing offers remarkable benefits and safeguards against potential harm. This article delves deep into the pervasive role of pH testing in ensuring a healthier, safer, and more sustainable everyday existence.
pH: The Acid-Alkali Balance
Before diving into the applications of pH testing, it's crucial to grasp the fundamental concept of pH itself. The pH scale ranges from 0 to 14, with 7 representing neutrality. Values below 7 indicate acidity (e.g., lemon juice, stomach acid), while values above 7 signify alkalinity (e.g., baking soda, seawater). Each whole number change on the scale represents a tenfold difference in acidity or alkalinity. This seemingly simple scale holds profound implications across numerous areas.
The Importance of Maintaining Optimal pH Levels
Maintaining the correct pH level is critical for numerous processes and systems. A slight deviation from the optimal range can have significant consequences, ranging from minor inconveniences to severe health problems and environmental damage. The significance lies in the fact that pH influences chemical reactions, enzymatic activity, and the overall stability of various systems.
pH Testing in Food and Drink
The culinary world heavily relies on pH testing for ensuring food safety, quality, and optimal taste. Let's explore some key applications:
1. Food Preservation and Safety:
- Preventing Spoilage: Many food spoilage microorganisms thrive within specific pH ranges. By monitoring and controlling the pH of food products during processing and storage, manufacturers can effectively inhibit microbial growth and extend shelf life. This is particularly vital for canned goods, jams, and other preserved foods.
- Safeguarding Against Bacterial Growth: Certain bacteria, such as E. coli and Salmonella, cannot survive in highly acidic environments. pH testing plays a critical role in ensuring that foods like pickles and sauerkraut have reached the appropriate level of acidity to prevent the growth of these harmful bacteria.
- Maintaining Quality and Texture: The pH of food directly influences its texture and overall quality. In the production of dairy products like yogurt and cheese, precise pH control is essential for achieving the desired consistency and preventing spoilage.
2. Winemaking and Brewing:
- Controlling Fermentation: pH levels significantly affect the fermentation process in winemaking and brewing. Maintaining optimal pH is essential for healthy yeast activity and the production of high-quality beverages. Testing throughout the process allows winemakers and brewers to adjust acidity levels as needed.
- Flavor Enhancement: The pH of wine and beer directly influences their flavor profile. Precise pH control allows for the creation of beverages with desirable taste characteristics.
3. Home Cooking:
- Baking: The pH of ingredients affects the rising and texture of baked goods. Understanding the pH of ingredients can be helpful in troubleshooting baking problems.
- Pickling: Precise pH control is crucial for safe and successful pickling, ensuring the preservation of vegetables and a desirable sour taste.
pH Testing in Healthcare
The human body maintains a precise pH balance across various systems. Deviations from these optimal ranges can lead to health complications. pH testing plays a crucial role in diagnosis and treatment in several medical areas:
1. Blood pH:
- Acid-Base Balance: Maintaining a precise blood pH (slightly alkaline, around 7.4) is crucial for the proper functioning of various bodily systems. Acidosis (low blood pH) and alkalosis (high blood pH) can lead to serious health consequences, necessitating prompt medical intervention. Arterial blood gas analysis routinely measures blood pH to identify and manage these imbalances.
- Diagnosing Medical Conditions: Abnormal blood pH can be an indicator of various underlying medical conditions, including kidney disease, lung disease, and diabetes. Regular blood tests can aid in the early diagnosis and treatment of these ailments.
2. Urine pH:
- Kidney Function Assessment: Urine pH provides valuable information about kidney function and the body's ability to regulate acid-base balance. Abnormal urine pH can indicate kidney stones, urinary tract infections, or metabolic disorders.
- Monitoring Medication Effectiveness: Urine pH testing can be used to monitor the effectiveness of certain medications.
3. Other Bodily Fluids:
- Gastric pH: Measuring the pH of stomach acid can help diagnose conditions like gastroesophageal reflux disease (GERD) and assess the effectiveness of antacid medications.
- Vaginal pH: Testing vaginal pH can assist in diagnosing yeast infections and bacterial vaginosis.
pH Testing in Environmental Monitoring
pH testing is indispensable for environmental monitoring and protection. It's crucial for assessing water quality, soil health, and the impact of pollution.
1. Water Quality:
- Assessing Water Purity: pH levels in water sources indicate water purity and the presence of pollutants. Acid rain, for example, significantly lowers the pH of lakes and rivers, harming aquatic life.
- Monitoring Water Treatment Processes: pH testing is crucial in water treatment plants to ensure that water is properly treated and safe for consumption.
- Protecting Aquatic Ecosystems: Monitoring the pH of aquatic environments is vital for maintaining the health of fish and other aquatic organisms.
2. Soil Health:
- Optimizing Plant Growth: The pH of soil significantly influences nutrient availability for plants. Testing soil pH allows farmers and gardeners to amend the soil with appropriate materials to optimize plant growth.
- Assessing Soil Quality: Soil pH reflects the overall health and composition of the soil. Abnormal pH levels can indicate soil contamination or degradation.
3. Pollution Monitoring:
- Acid Rain Detection: Monitoring the pH of rainwater helps track acid rain patterns and their impact on the environment.
- Industrial Waste Management: pH testing is essential for monitoring industrial wastewater to ensure it meets environmental regulations before discharge.
pH Testing Methods
Various methods exist for measuring pH, ranging from simple home testing kits to sophisticated laboratory equipment.
1. pH Indicator Strips/Papers:
These are inexpensive and easy-to-use strips that change color based on the pH of the solution. While not as precise as other methods, they provide a quick and convenient way to obtain a rough estimate of pH.
2. pH Meters:
Digital pH meters are more precise instruments that provide a numerical reading of the pH level. They are widely used in laboratories and industrial settings. Calibration is essential for accurate readings.
3. Titration:
Titration is a laboratory technique that involves adding a solution of known concentration to a solution of unknown concentration until a specific reaction occurs. This method is more complex but can provide highly accurate pH measurements.
Conclusion: The Ubiquitous Importance of pH Testing
The importance of pH testing permeates various facets of our everyday lives. From ensuring the safety and quality of our food and drink to safeguarding our health and protecting our environment, pH testing plays a crucial role in maintaining a better quality of life. Understanding the principles of pH and utilizing appropriate testing methods empowers us to make informed decisions, contributing to a healthier, safer, and more sustainable future. The seemingly simple act of pH measurement is, in reality, an unsung hero underpinning many critical aspects of our daily existence. Embracing this fundamental concept and its applications can significantly improve our well-being and contribute to a healthier planet.
Latest Posts
Latest Posts
-
1 Mole Of Carbon In Grams
Apr 01, 2025
-
What Is The Charge On A Sulfide Ion
Apr 01, 2025
-
What Is Not A Function Of The Lymphatic System
Apr 01, 2025
-
Who Has Hemophilia In The Pedigree That Is Shown
Apr 01, 2025
-
A Chord That Passes Through The Center Of A Circle
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Explain The Importance Of Ph Testing In Everyday Life . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
