What Is The Molecular Mass Of Caco3
News Leon
Mar 17, 2025 · 5 min read
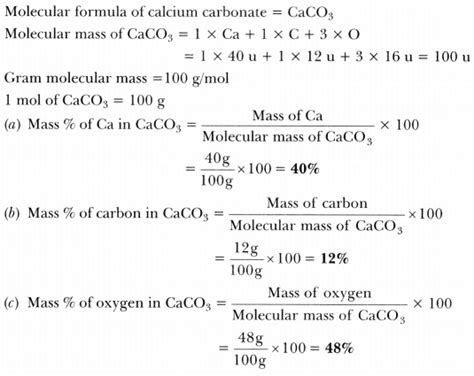
Table of Contents
What is the Molecular Mass of CaCO3? A Deep Dive into Calcium Carbonate
Calcium carbonate (CaCO3) is a ubiquitous compound found in various forms throughout the natural world and extensively used in numerous industrial applications. Understanding its molecular mass is fundamental to various scientific and engineering calculations. This article will explore the calculation of CaCO3's molecular mass, delve into its applications, and touch upon its significance in different fields.
Understanding Molecular Mass
Before calculating the molecular mass of CaCO3, it's crucial to understand the concept. Molecular mass, also known as molecular weight, represents the total mass of all atoms in a molecule. It's expressed in atomic mass units (amu) or Daltons (Da). The mass of each atom is determined by its atomic number and isotopic abundance. For calculations, we typically use the standard atomic weights listed in the periodic table.
Calculating the Molecular Mass of CaCO3
To determine the molecular mass of CaCO3, we need the atomic masses of its constituent elements: Calcium (Ca), Carbon (C), and Oxygen (O). The standard atomic weights are approximately:
- Calcium (Ca): 40.08 amu
- Carbon (C): 12.01 amu
- Oxygen (O): 16.00 amu
CaCO3 contains:
- 1 Calcium atom (Ca)
- 1 Carbon atom (C)
- 3 Oxygen atoms (O)
Therefore, the molecular mass of CaCO3 is calculated as follows:
(1 × Atomic mass of Ca) + (1 × Atomic mass of C) + (3 × Atomic mass of O) = (1 × 40.08 amu) + (1 × 12.01 amu) + (3 × 16.00 amu) = 100.09 amu
Therefore, the molecular mass of CaCO3 is approximately 100.09 amu.
Significance of the Molecular Mass of CaCO3
Knowing the molecular mass of CaCO3 is crucial for various reasons:
-
Stoichiometric Calculations: In chemical reactions, the molecular mass allows for precise calculations of reactant and product quantities. This is essential in industrial processes and laboratory experiments.
-
Molar Mass Calculations: The molecular mass is numerically equivalent to the molar mass, which is the mass of one mole of a substance (approximately 6.022 x 10²³ particles). This is critical for converting between mass and moles, essential in many chemical analyses and calculations.
-
Concentration Determinations: In solutions, knowing the molecular mass helps determine concentrations in terms of molarity (moles per liter) and other concentration units.
-
Material Science and Engineering: In materials science and engineering, the molecular mass plays a vital role in understanding material properties, predicting behavior, and designing new materials. For example, the molecular mass influences the crystal structure and mechanical properties of calcium carbonate-based materials.
-
Environmental Science: Understanding the molecular mass of CaCO3 is crucial for assessing its role in environmental processes, such as carbon sequestration and ocean acidification. Accurate calculations are needed for modeling and predicting these processes.
-
Geological Studies: In geology, the molecular mass is essential for analyzing mineral compositions and understanding geological formations. It helps determine the relative abundance of calcium carbonate in rocks and sediments.
-
Pharmaceutical Applications: Calcium carbonate is used as a dietary supplement and in pharmaceutical formulations. Precise knowledge of the molecular mass is vital for dosage calculations and formulation development.
Applications of CaCO3
Calcium carbonate's widespread use stems from its abundance, relatively low cost, and diverse properties. Its applications span numerous industries:
1. Construction and Building Materials:
- Cement Production: CaCO3 is a key ingredient in cement production, providing calcium oxide (lime) after calcination. This lime reacts with other components to form the cement matrix.
- Limestone: Naturally occurring forms of CaCO3 (limestone) are used directly as a construction material, in aggregate, and as a base for roads.
- Plaster and Mortar: Calcium carbonate is used in plaster and mortar mixes to bind the components together.
2. Agriculture:
- Soil Amendment: Ground limestone (CaCO3) is commonly used to amend acidic soils, raising their pH to optimal levels for crop growth.
- Animal Feed: Calcium carbonate serves as a calcium supplement in animal feed, essential for bone development and other physiological processes.
3. Chemical Industry:
- Production of Calcium Oxide (Lime): Thermal decomposition of CaCO3 produces CaO, used extensively in various industrial applications.
- Paper Industry: CaCO3 serves as a filler and coating material in paper production, improving brightness and opacity.
- Pigments and Paints: CaCO3 is used as a pigment in paints and coatings, providing opacity and improving color brightness.
4. Food Industry:
- Food Additive: Calcium carbonate acts as an anticaking agent, preventing clumping in powdered foods.
- Dietary Supplement: It's used as a calcium supplement in various food products and dietary supplements.
5. Pharmaceuticals and Healthcare:
- Antacid: CaCO3 is a common ingredient in antacids due to its ability to neutralize stomach acid.
- Calcium Supplement: It serves as a source of dietary calcium for preventing and treating calcium deficiencies.
6. Environmental Applications:
- Water Treatment: CaCO3 can be used in water treatment processes for controlling acidity and improving water quality.
- Carbon Capture: Research is exploring the use of CaCO3 for carbon capture and storage, mitigating the impact of greenhouse gas emissions.
Variations in Molecular Mass: Isotopes
While the standard atomic weight provides an accurate approximation for most calculations, it's important to note that the molecular mass can vary slightly depending on the isotopic composition of the elements. Natural calcium, for example, consists of several isotopes (⁴⁰Ca, ⁴²Ca, ⁴³Ca, ⁴⁴Ca, ⁴⁶Ca, ⁴⁸Ca), each with a different mass. Similarly, carbon and oxygen have their isotopes. The standard atomic weights reflect the average mass considering the abundance of these isotopes. For highly precise calculations, the exact isotopic composition of the sample needs to be considered to determine a more precise molecular mass.
Conclusion:
The molecular mass of CaCO3, approximately 100.09 amu, is a fundamental property with significant implications across numerous scientific disciplines and industries. Its accurate calculation is crucial for stoichiometric calculations, concentration determinations, and understanding material properties. The widespread applications of CaCO3, from construction to pharmaceuticals, highlight its importance in our daily lives. While the standard atomic weights provide an accurate enough value for many applications, the understanding of isotopic variations allows for even more precise calculations when required. Further research into its properties and potential applications continues to reveal its versatility and ongoing importance.
Latest Posts
Latest Posts
-
A Current Of One Ampere Is Passed Through
Mar 18, 2025
-
Difference Between Earthing And Grounding And Neutral
Mar 18, 2025
-
What Is The Molar Mass Of Calcium Carbonate Caco3
Mar 18, 2025
-
How Many Lines Of Symmetry Does The Square Have
Mar 18, 2025
-
What Is The Molar Mass Of Nahco3
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molecular Mass Of Caco3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
