What Is The Molar Mass Of S
News Leon
Mar 25, 2025 · 6 min read
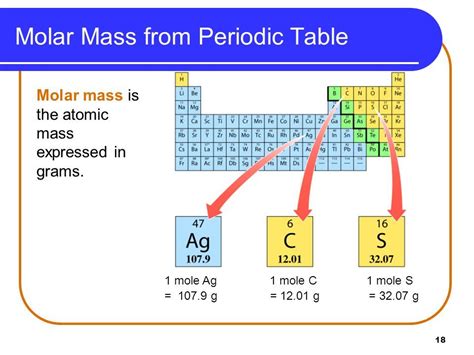
Table of Contents
What is the Molar Mass of Sulfur (S)? A Deep Dive into Atomic Weight and Applications
Sulfur, a vibrant yellow nonmetal, plays a crucial role in various aspects of our lives, from the formation of proteins to the production of sulfuric acid. Understanding its properties, especially its molar mass, is fundamental to numerous scientific and industrial applications. This comprehensive guide delves into the concept of molar mass, specifically focusing on sulfur (S), exploring its calculation, significance, and practical implications across different fields.
Understanding Molar Mass
Before we delve into the molar mass of sulfur, let's establish a clear understanding of the concept itself. Molar mass represents the mass of one mole of a substance. A mole is a fundamental unit in chemistry, defined as the amount of a substance containing Avogadro's number (approximately 6.022 x 10<sup>23</sup>) of elementary entities (atoms, molecules, ions, etc.). Therefore, the molar mass essentially tells us the mass of 6.022 x 10<sup>23</sup> atoms or molecules of a particular substance. It is typically expressed in grams per mole (g/mol).
The molar mass of an element is numerically equal to its atomic weight (or standard atomic weight) expressed in atomic mass units (amu). The atomic weight is the weighted average of the masses of all naturally occurring isotopes of an element, taking into account their relative abundances. This means that the molar mass isn't a fixed, absolute value for an element, as it accounts for the natural variability in isotopic composition.
Calculating the Molar Mass of Sulfur (S)
Sulfur exists in various isotopic forms, the most common being <sup>32</sup>S, <sup>33</sup>S, <sup>34</sup>S, and <sup>36</sup>S. Each isotope contributes to the overall atomic weight, weighted by its natural abundance. The standard atomic weight of sulfur, as reported by the IUPAC (International Union of Pure and Applied Chemistry), is approximately 32.065 g/mol. This value represents the weighted average of the masses of all naturally occurring sulfur isotopes. Therefore, the molar mass of sulfur is also 32.065 g/mol.
This means that one mole of sulfur atoms has a mass of approximately 32.065 grams. This seemingly simple number holds immense importance in various stoichiometric calculations and chemical analyses.
Importance of Precision in Molar Mass
While 32 g/mol is a commonly used approximation, using the more precise value of 32.065 g/mol is crucial for accurate calculations, especially in situations requiring high precision, such as:
-
Analytical Chemistry: In quantitative analyses, using the precise molar mass ensures accurate determination of the amount of sulfur in a sample. Errors stemming from using an approximate value can significantly impact the results, potentially leading to inaccurate conclusions.
-
Industrial Processes: In industrial settings where sulfur is used in large quantities, even small discrepancies in molar mass calculations can affect the efficiency and yield of processes. Precise molar mass values ensure accurate stoichiometric control and optimal resource utilization.
-
Research and Development: In scientific research, precise molar mass is essential for accurate modelling and simulations, ensuring the reliability and reproducibility of experimental results.
Applications of Sulfur and the Significance of its Molar Mass
Sulfur's versatility stems from its ability to form various compounds, influencing its wide range of applications. The accurate determination of its molar mass plays a critical role in understanding and utilizing these applications:
1. Sulfuric Acid Production
Sulfuric acid (H₂SO₄), one of the most important industrial chemicals globally, is primarily produced through the contact process. The molar mass of sulfur is fundamental in determining the stoichiometric ratios of reactants and products in this process, ensuring efficient production and minimizing waste. Understanding the molar mass allows for precise control over the reaction conditions, optimizing yield and minimizing energy consumption.
2. Vulcanization of Rubber
Sulfur plays a pivotal role in vulcanizing rubber, a process that transforms natural rubber from a sticky, elastic material into a durable, resilient substance suitable for various applications. The molar mass of sulfur influences the extent of cross-linking between polymer chains, directly impacting the final properties of the vulcanized rubber. The amount of sulfur used is carefully controlled based on its molar mass to achieve desired characteristics like tensile strength, elasticity, and resistance to wear and tear.
3. Production of Fertilizers
Elemental sulfur is a key component in the production of various fertilizers, notably ammonium sulfate [(NH₄)₂SO₄] and superphosphates. Accurate calculation of sulfur's molar mass is crucial in determining the precise amounts of sulfur needed to produce these fertilizers, ensuring the right nutrient balance for optimal plant growth.
4. Pharmaceuticals and Medicine
Sulfur and its compounds find applications in several pharmaceutical formulations. For example, sulfur-containing drugs are used in the treatment of various skin conditions. Understanding the molar mass of sulfur enables accurate dosing and formulation of these medications, ensuring their safety and effectiveness.
5. Matches and Fireworks
Sulfur's combustible nature makes it a crucial component in the manufacturing of matches and fireworks. The molar mass helps determine the appropriate amounts needed to achieve the desired explosive or combustion characteristics, ensuring both safety and performance.
Beyond Elemental Sulfur: Molar Mass in Sulfur Compounds
The molar mass of sulfur is not only important for elemental sulfur but also essential when dealing with various sulfur-containing compounds. Calculating the molar mass of these compounds requires adding the atomic masses of all constituent atoms, weighted according to the number of atoms of each element present in the molecule.
For example, to calculate the molar mass of sulfuric acid (H₂SO₄):
- Hydrogen (H): 1.008 g/mol x 2 = 2.016 g/mol
- Sulfur (S): 32.065 g/mol x 1 = 32.065 g/mol
- Oxygen (O): 15.999 g/mol x 4 = 63.996 g/mol
Total Molar Mass of H₂SO₄: 2.016 + 32.065 + 63.996 = 98.077 g/mol
This calculation demonstrates how the molar mass of sulfur is a fundamental component in determining the molar mass of its compounds, crucial for various chemical calculations and applications.
Conclusion: The Unsung Hero of Chemical Calculations
The molar mass of sulfur, seemingly a simple numerical value, is a cornerstone of numerous chemical calculations and industrial processes. Its precise determination (32.065 g/mol) is crucial for accuracy in various fields, from industrial production to pharmaceutical development and scientific research. Understanding its significance highlights the importance of precision and attention to detail in chemical computations and underscores the fundamental role of molar mass in our understanding and application of this vital element. The accurate use of molar mass ensures efficient production, accurate analyses, and the development of safe and effective products that rely on sulfur's unique properties.
Latest Posts
Latest Posts
-
A Homogeneous Mixture Of Two Or More Substances Is A
Mar 26, 2025
-
In Which Stage Of Meiosis Crossing Over Occurs
Mar 26, 2025
-
Which Of The Following Is Not A Lymphoid Organ
Mar 26, 2025
-
What Is The Reason For Doing A Test Cross
Mar 26, 2025
-
Is The Following Relation A Function
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of S . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
