A Homogeneous Mixture Of Two Or More Substances Is A
News Leon
Mar 26, 2025 · 6 min read
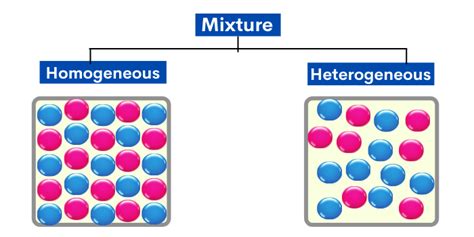
Table of Contents
A Homogeneous Mixture of Two or More Substances Is a Solution: A Deep Dive
A homogeneous mixture is a type of mixture in which the composition is uniform throughout the mixture. This means that the different components of the mixture are evenly distributed and cannot be easily distinguished visually or by other simple means. The most common and quintessential example of a homogeneous mixture is a solution. This article will delve deep into the nature of homogeneous mixtures, focusing specifically on solutions and their properties, exploring diverse examples, and discussing the crucial role they play in various scientific fields and everyday life.
Understanding Homogeneous Mixtures: A Definition
Before we dive into the specifics of solutions, let's solidify our understanding of homogeneous mixtures. A key characteristic is the uniformity of the composition. No matter where you take a sample from the mixture, it will have the same proportions of each component. This is in contrast to a heterogeneous mixture, where components are not uniformly distributed and different samples may have varying compositions (think of sand and water, for example).
Key features of homogeneous mixtures:
- Uniform composition: The components are evenly dispersed at a molecular or ionic level.
- Single phase: Homogeneous mixtures exist in a single phase (solid, liquid, or gas), unlike heterogeneous mixtures which often display multiple phases.
- Invisible components: The individual components are not readily visible to the naked eye. This requires magnification to distinguish individual components, which isn’t always possible, depending on the mixture.
- Easily separated by physical means: In some cases (though not all), the components can be separated using physical methods like filtration, distillation, or evaporation. This separation exploits differences in physical properties of the components, such as boiling points or solubility.
Solutions: The Quintessential Homogeneous Mixture
While all solutions are homogeneous mixtures, not all homogeneous mixtures are solutions. A solution is a special type of homogeneous mixture where one substance, the solute, dissolves completely into another substance, the solvent, resulting in a single, uniform phase. The solute is typically present in a smaller amount than the solvent.
Components of a solution:
- Solute: The substance being dissolved. This can be a solid, liquid, or gas.
- Solvent: The substance doing the dissolving. This is typically a liquid, but can also be a solid or a gas.
Examples of Solutions:
The world around us is full of solutions! Here are some examples categorized by the state of matter of the solvent and solute:
Liquid Solutions:
- Saltwater: Salt (NaCl) is the solute, and water (H₂O) is the solvent. This is a classic example used frequently in chemistry demonstrations and experiments.
- Sugar water: Sugar (sucrose) dissolved in water. This is another common example, illustrating the solubility of solid solutes in liquid solvents.
- Air: Although we don't usually think of air as a solution, it’s a gaseous solution comprising primarily nitrogen and oxygen, with trace amounts of other gases. Nitrogen acts as the solvent, dissolving the other gaseous components.
- Vinegar: Acetic acid (a liquid) dissolved in water. Vinegar's characteristic sour taste comes from the dissolved acetic acid.
- Alcoholic beverages: Ethanol (a liquid) dissolved in water. The percentage of ethanol determines the alcoholic strength of the drink.
Solid Solutions (Alloys):
- Brass: A mixture of copper and zinc. Brass is harder than pure copper and has a more appealing golden color. This is an example of a solid solution where both the solute and solvent are solids.
- Steel: Iron with added carbon, and potentially other elements like chromium or nickel. The added elements modify the properties of the iron, increasing hardness and strength. This demonstrates the alteration of properties by the addition of solutes.
- Sterling silver: Silver alloyed with a small percentage of copper. The addition of copper enhances the strength and durability of the silver.
Gas Solutions:
- Air (revisited): The gases in the air are completely miscible, forming a homogeneous gaseous solution. The differing boiling points of the components allow for their separation through fractional distillation, a technique that exploits the difference in boiling points to separate a mixture.
Factors Affecting Solubility
The extent to which a solute dissolves in a solvent depends on several factors:
- Nature of the solute and solvent: "Like dissolves like" is a fundamental principle. Polar solvents tend to dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. For example, water (a polar solvent) readily dissolves salt (a polar compound), but not oil (a nonpolar substance).
- Temperature: Generally, increasing the temperature increases the solubility of solids and liquids in liquids. However, the solubility of gases in liquids decreases with increasing temperature. This is why opening a warm soda releases more carbon dioxide.
- Pressure: Pressure has a significant effect on the solubility of gases in liquids. Increasing pressure increases the solubility of gases. This principle is exploited in carbonated beverages, where carbon dioxide is dissolved under pressure.
- Surface area of the solute: A finely divided solute will dissolve faster than a large lump because of the increased surface area available for interaction with the solvent.
Concentration of Solutions
The concentration of a solution describes the amount of solute dissolved in a given amount of solvent or solution. Several ways express concentration:
- Molarity (M): Moles of solute per liter of solution.
- Molality (m): Moles of solute per kilogram of solvent.
- Mass percent (%): Grams of solute per 100 grams of solution.
- Volume percent (% v/v): Milliliters of solute per 100 milliliters of solution.
- Parts per million (ppm) and parts per billion (ppb): Used for very dilute solutions.
Applications of Homogeneous Mixtures and Solutions
Homogeneous mixtures and solutions are fundamental in numerous areas of science, technology, and everyday life. Some examples include:
- Medicine: Many drugs are administered as solutions, ensuring even distribution and controlled dosage. Intravenous fluids are solutions designed to maintain hydration and electrolyte balance in the body.
- Industry: Chemical processes often involve solutions as reactants or solvents. Electroplating utilizes solutions to deposit thin layers of metal onto other surfaces.
- Agriculture: Fertilizers are frequently applied as solutions to provide essential nutrients to plants. Pesticides and herbicides are also often delivered in solution form for effective distribution.
- Food science: Many food products are solutions, such as fruit juices, soft drinks, and sauces. The solubility of different components affects texture, taste, and shelf life.
- Environmental science: Understanding the solubility of pollutants in water and soil is crucial for assessing environmental risks and developing remediation strategies.
Separating Components of Solutions
While the components of a homogeneous mixture are evenly distributed, they can often be separated using various techniques that exploit differences in their physical properties:
- Evaporation: Used to separate a soluble solid from a liquid solvent. The solvent evaporates, leaving the solid behind.
- Distillation: Used to separate liquids with different boiling points. The liquid with the lower boiling point evaporates first and is collected separately.
- Chromatography: Used to separate mixtures based on the different affinities of the components for a stationary and mobile phase. This is particularly useful for separating complex mixtures.
- Crystallization: A technique used to separate a solid solute from a solution by altering conditions to reduce its solubility. This often leads to the formation of pure crystals.
Conclusion
Homogeneous mixtures, particularly solutions, are ubiquitous in our world. Their properties and behavior are governed by various factors, and understanding these principles is crucial in numerous scientific and technological applications. From the air we breathe to the medicines we take, homogeneous mixtures play an essential role in shaping our lives. Further exploration of this fundamental concept opens doors to a deeper understanding of the chemical and physical processes that govern our universe. The study of solutions is a continuous journey of discovery, unveiling the intricate interactions between different substances and their profound impact on the world around us.
Latest Posts
Latest Posts
-
Why Is The Market Demand Curve Downward Sloping
Mar 29, 2025
-
Are Cheek Cells Prokaryotic Or Eukaryotic
Mar 29, 2025
-
When A Lysosome Fuses With A Vacuole
Mar 29, 2025
-
In The Figure The Four Particles Form A Square
Mar 29, 2025
-
2 Km Equals How Many Centimeters
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about A Homogeneous Mixture Of Two Or More Substances Is A . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
