What Is The Molar Mass Of Nh4 2co3
News Leon
Mar 24, 2025 · 5 min read
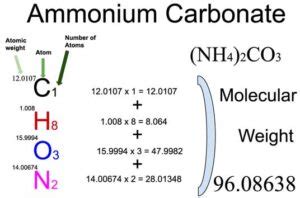
Table of Contents
What is the Molar Mass of (NH₄)₂CO₃? A Deep Dive into Ammonium Carbonate
Ammonium carbonate, (NH₄)₂CO₃, is a fascinating inorganic salt with a wide range of applications, from baking to the production of other chemicals. Understanding its molar mass is crucial for various chemical calculations and applications. This in-depth article will not only calculate the molar mass of ammonium carbonate but will also delve into the concept of molar mass, its significance, and practical applications.
Understanding Molar Mass: The Foundation
Before calculating the molar mass of (NH₄)₂CO₃, it's essential to grasp the fundamental concept of molar mass. Molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of elementary entities, which could be atoms, molecules, ions, or other specified particles. The molar mass is expressed in grams per mole (g/mol).
Knowing the molar mass is crucial for various chemical calculations, including:
- Stoichiometry: Determining the quantities of reactants and products in a chemical reaction.
- Concentration calculations: Calculating the concentration of solutions in molarity (moles per liter).
- Titrations: Determining the unknown concentration of a solution.
- Gas law calculations: Utilizing the ideal gas law to predict the behavior of gases.
The molar mass of an element is simply its atomic weight (found on the periodic table) expressed in grams per mole. For example, the molar mass of carbon (C) is 12.01 g/mol, and the molar mass of oxygen (O) is 16.00 g/mol.
Calculating the Molar Mass of (NH₄)₂CO₃: A Step-by-Step Approach
To calculate the molar mass of (NH₄)₂CO₃, we need to consider the molar mass of each element present in the compound and the number of atoms of each element. The formula indicates that one molecule of ammonium carbonate contains:
- 2 Nitrogen (N) atoms: The atomic weight of nitrogen is approximately 14.01 g/mol.
- 8 Hydrogen (H) atoms: The atomic weight of hydrogen is approximately 1.01 g/mol.
- 1 Carbon (C) atom: The atomic weight of carbon is approximately 12.01 g/mol.
- 3 Oxygen (O) atoms: The atomic weight of oxygen is approximately 16.00 g/mol.
Therefore, the molar mass of (NH₄)₂CO₃ is calculated as follows:
(2 x 14.01 g/mol) + (8 x 1.01 g/mol) + (1 x 12.01 g/mol) + (3 x 16.00 g/mol) = 96.09 g/mol
Therefore, the molar mass of (NH₄)₂CO₃ is approximately 96.09 g/mol.
Understanding the Significance of Accurate Calculation
The accuracy of the molar mass calculation is crucial for obtaining reliable results in various chemical experiments and calculations. Even small errors in the atomic weights or the number of atoms can lead to significant discrepancies in the final results. Therefore, always refer to an updated periodic table for the most accurate atomic weights.
Practical Applications of Ammonium Carbonate and its Molar Mass
Ammonium carbonate, despite its somewhat pungent odor, finds numerous applications in various fields:
1. Baking and Food Industry
Historically, ammonium carbonate was used as a leavening agent in baking, particularly in some traditional European pastries. It decomposes at relatively low temperatures, releasing ammonia and carbon dioxide gases, which cause the batter or dough to rise. However, its use has largely been replaced by more modern leavening agents due to its strong smell.
Understanding its molar mass is crucial for bakers who wish to precisely control the amount of leavening agent in their recipes.
2. Pharmaceutical Industry
Ammonium carbonate has been used in some pharmaceutical preparations, though its application is limited due to its odor. It has been used in expectorants and other medications. Accurate molar mass calculations are essential for precise formulation of medications.
3. Production of Other Chemicals
Ammonium carbonate serves as a precursor for the synthesis of other ammonium salts and other chemicals. Knowledge of its molar mass is vital in optimizing these chemical reactions and determining the precise quantities of reactants required.
4. Fertilizers and Agriculture
Ammonium carbonate can also be a source of nitrogen, a crucial nutrient for plant growth. However, its use as a fertilizer is limited due to its volatility and potential for odor problems.
Precise calculations involving its molar mass allow agricultural scientists to assess its effectiveness as a nitrogen source in specific applications.
Beyond the Basics: Further Exploration of Related Concepts
Understanding the molar mass of (NH₄)₂CO₃ leads to a deeper understanding of related concepts in chemistry:
1. Mole Concept and Avogadro's Number
The mole concept is fundamental to quantitative chemistry. Avogadro's number is a constant that relates the mass of a substance to the number of particles it contains. This understanding is essential for interpreting experimental data and performing calculations related to chemical reactions.
2. Stoichiometry and Chemical Reactions
Stoichiometry allows us to predict the amounts of reactants and products in chemical reactions based on their molar masses and the balanced chemical equation. This is crucial for industrial processes and laboratory experiments.
3. Solution Chemistry and Molarity
Understanding the molar mass is vital for preparing solutions of specific concentrations, expressed as molarity (moles per liter). Molarity is a widely used unit in chemistry and biochemistry.
4. Gas Laws and Ideal Gases
For gases, the molar mass is directly related to their density and behavior as described by ideal gas laws. Accurate molar mass calculations are needed for precise predictions of gas behavior.
Conclusion: The Importance of Molar Mass in Chemistry
The molar mass of (NH₄)₂CO₃, approximately 96.09 g/mol, is not just a simple numerical value; it's a fundamental piece of information that unlocks a deeper understanding of this compound's behavior and applications. From baking to pharmaceutical production to agricultural use, the accurate calculation and application of molar mass ensures precise control and efficient use of ammonium carbonate and other chemical substances. Mastering the concept of molar mass is crucial for any aspiring chemist or anyone working in fields that involve chemical processes. The significance extends beyond simple calculations; it provides the foundation for understanding and manipulating matter at a molecular level. Further exploration of related concepts will enhance the understanding of chemical reactions and their impact on various scientific and technological advancements.
Latest Posts
Latest Posts
-
Sodium Is A Solid Liquid Or Gas
Mar 25, 2025
-
In The Figure Projectile Particle 1 Is An Alpha
Mar 25, 2025
-
Si Unit Of Measurement For Acceleration
Mar 25, 2025
-
What Is The Basic Unit For Distance
Mar 25, 2025
-
Cell Type Not Found In Areolar Tissue
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Nh4 2co3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
