In The Figure Projectile Particle 1 Is An Alpha
News Leon
Mar 25, 2025 · 6 min read
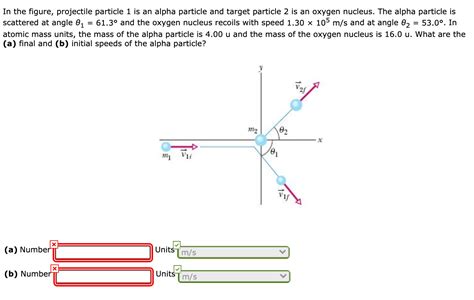
Table of Contents
- In The Figure Projectile Particle 1 Is An Alpha
- Table of Contents
- In the Figure, Projectile Particle 1 is an Alpha: Unveiling the Physics of Alpha Particle Scattering
- Key Properties of Alpha Particles:
- The Experimental Setup:
- Unexpected Results and the Nuclear Model:
- Implications of the Experiment:
- Calculating Scattering Angles:
- Rutherford Backscattering Spectrometry (RBS):
- Nuclear Physics Research:
- Other Applications:
- Latest Posts
- Latest Posts
- Related Post
In the Figure, Projectile Particle 1 is an Alpha: Unveiling the Physics of Alpha Particle Scattering
This article delves into the fascinating world of alpha particle scattering, specifically focusing on scenarios where particle 1 in a given figure is an alpha particle. We will explore the underlying physics, the historical significance of alpha scattering experiments (particularly Rutherford's gold foil experiment), and the implications for our understanding of atomic structure. We'll also touch upon modern applications and advancements in this field.
Understanding Alpha Particles:
Before diving into the specifics of scattering experiments, let's establish a firm understanding of alpha particles. An alpha particle (α-particle) is essentially a helium nucleus, consisting of two protons and two neutrons. This gives it a +2 charge and a relatively large mass compared to other subatomic particles. Because of its mass and charge, the alpha particle interacts strongly with matter, making it a useful probe for studying atomic structure.
Key Properties of Alpha Particles:
- Charge: +2e (where 'e' is the elementary charge)
- Mass: Approximately 4 atomic mass units (amu)
- Velocity: Varies depending on the source and acceleration; typically high velocity in scattering experiments.
- Penetration Power: Relatively low compared to beta or gamma radiation, easily stopped by a thin sheet of paper or a few centimeters of air.
- Ionizing Power: High, due to its charge and mass, leading to significant ionization events as it passes through matter.
Rutherford's Gold Foil Experiment and the Birth of the Nuclear Model:
The most famous experiment involving alpha particle scattering is undoubtedly Ernest Rutherford's gold foil experiment, conducted in 1909 by his team (Hans Geiger and Ernest Marsden). This landmark experiment revolutionized our understanding of the atom.
The Experimental Setup:
Rutherford's team bombarded a thin gold foil with a beam of alpha particles emitted from a radioactive source. A fluorescent screen surrounding the foil detected the scattered alpha particles. They expected the alpha particles to pass straight through the foil with minimal deflection, based on the then-current "plum pudding" model of the atom.
Unexpected Results and the Nuclear Model:
The results were astonishing. While most alpha particles did pass straight through, a significant number were deflected at large angles, and some even bounced back directly towards the source. This unexpected scattering pattern could not be explained by the plum pudding model. Rutherford concluded that the atom's positive charge was not distributed uniformly but concentrated in a tiny, dense region at the center—the nucleus.
Implications of the Experiment:
This groundbreaking experiment led to the development of the nuclear model of the atom, a model that continues to form the basis of our understanding of atomic structure. The experiment showed:
- The atom is mostly empty space: Most alpha particles passed through the foil undeflected, indicating that the atom is mostly empty space.
- The atom has a small, dense, positively charged nucleus: The large-angle scattering of some alpha particles revealed the presence of a tiny, dense, positively charged nucleus at the atom's center.
- Electrons orbit the nucleus: The negatively charged electrons were thought to orbit the nucleus to balance the positive charge.
Mathematical Description of Alpha Particle Scattering:
The scattering of alpha particles can be described mathematically using Coulomb's law, which governs the interaction between charged particles. The force between the alpha particle (charge +2e) and the nucleus (charge +Ze, where Z is the atomic number) is given by:
F = (k * (2e) * (Ze)) / r²
Where:
- F is the electrostatic force
- k is Coulomb's constant
- e is the elementary charge
- Z is the atomic number of the target nucleus
- r is the distance between the alpha particle and the nucleus
This force leads to a deflection of the alpha particle's trajectory, the magnitude of which depends on the impact parameter (the distance of closest approach if the particle were to travel undeflected), the velocity of the alpha particle, and the charge of the nucleus.
Calculating Scattering Angles:
Using classical mechanics and Coulomb's law, one can derive equations to predict the scattering angle (θ) as a function of the impact parameter and other relevant parameters. The probability of scattering at a particular angle can also be calculated, which is crucial for interpreting experimental data. More complex calculations involving quantum mechanics are needed for highly accurate predictions.
Modern Applications of Alpha Particle Scattering:
While Rutherford's gold foil experiment was a pivotal moment, alpha particle scattering techniques continue to be relevant in modern physics and related fields.
Rutherford Backscattering Spectrometry (RBS):
RBS is a powerful analytical technique that uses alpha particle scattering to analyze the elemental composition and structure of materials. By measuring the energy and angle of scattered alpha particles, researchers can determine the types and amounts of atoms present in a sample. This technique finds applications in various fields, including materials science, semiconductor technology, and surface analysis.
Nuclear Physics Research:
Alpha particle scattering continues to play a role in nuclear physics research. By studying the scattering patterns of alpha particles from different nuclei, physicists can gather information about the size, shape, and structure of nuclei. This information is vital for our understanding of nuclear forces and nuclear reactions.
Other Applications:
Alpha particle scattering finds applications in other areas, such as:
- Medical imaging: While not as common as other techniques, alpha particles can be used in specific medical imaging modalities.
- Radiation therapy: The high ionizing power of alpha particles makes them potentially useful in targeted cancer therapy, though this is still an area of active research and development.
Challenges and Future Directions:
While alpha particle scattering has been incredibly valuable, there are challenges and opportunities for future development:
- Improving the precision of measurements: Further advancements in detector technology and data analysis techniques could lead to even more precise measurements of scattering angles and energies, enabling more detailed insights into atomic and nuclear structures.
- Developing new applications: Research continues to explore new applications of alpha particle scattering in various fields, including materials science, nanotechnology, and medicine.
- Investigating quantum effects: At very small distances and high energies, quantum effects become increasingly important, requiring advanced theoretical models to fully describe the scattering process.
Conclusion:
The study of alpha particle scattering, beginning with Rutherford's groundbreaking experiment, has been instrumental in shaping our understanding of atomic and nuclear structure. From revealing the existence of the atomic nucleus to providing powerful analytical tools for materials science and beyond, alpha particle scattering techniques continue to be invaluable in various scientific disciplines. The ongoing research in this field promises further advancements and novel applications in the years to come. The ongoing exploration of these scattering phenomena ensures that the legacy of Rutherford’s work continues to inspire and drive scientific discovery.
Latest Posts
Latest Posts
-
Halogen That Is Liquid At Room Temperature
Mar 27, 2025
-
The Basic Difference Between Macroeconomics And Microeconomics Is That
Mar 27, 2025
-
Boiling Water Is A Physical Change
Mar 27, 2025
-
The Name Of Al Oh 3 Is
Mar 27, 2025
-
What Is The Main Component Of Thin Filaments
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about In The Figure Projectile Particle 1 Is An Alpha . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
