Halogen That Is Liquid At Room Temperature
News Leon
Mar 27, 2025 · 6 min read
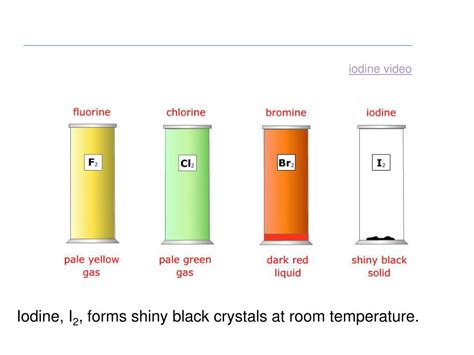
Table of Contents
- Halogen That Is Liquid At Room Temperature
- Table of Contents
- The Only Liquid Halogen at Room Temperature: Bromine – Properties, Production, and Applications
- Understanding Bromine's Unique Liquid State
- Intermolecular Forces and the Liquid State
- Key Properties of Bromine
- Bromine Production: From Brine to the Element
- 1. Brine Extraction and Pre-treatment
- 2. Oxidation of Bromide Ions
- 3. Bromine Extraction and Purification
- 4. Alternative Sources and Production Methods
- Diverse Applications of Bromine and its Compounds
- 1. Flame Retardants
- 2. Agricultural Chemicals
- 3. Water Treatment
- 4. Medical Applications
- 5. Dyes and Photography
- 6. Industrial Chemicals and Catalysts
- Environmental and Safety Concerns Associated with Bromine
- 1. Toxicity and Corrosiveness
- 2. Environmental Impact
- 3. Ozone Depletion Potential
- 4. Bioaccumulation and Biomagnification
- Future Prospects for Bromine and its Applications
- Sustainable Alternatives and Innovations
- Expanding Applications
- Conclusion: A Versatile but Carefully Managed Element
- Latest Posts
- Latest Posts
- Related Post
The Only Liquid Halogen at Room Temperature: Bromine – Properties, Production, and Applications
Bromine, a fascinating and crucial element, holds a unique distinction in the halogen family: it's the only halogen that exists as a liquid at room temperature. This characteristic, along with its diverse properties and applications, makes bromine a captivating subject for exploration. This comprehensive article delves into the world of bromine, examining its physical and chemical properties, production methods, prevalent applications, and safety considerations.
Understanding Bromine's Unique Liquid State
Unlike its fellow halogens – fluorine (F₂), chlorine (Cl₂), iodine (I₂), and astatine (At) – which exist as gases or solids at room temperature, bromine (Br₂) is a reddish-brown liquid. This unique property stems from the relatively weaker intermolecular forces (van der Waals forces) between its diatomic molecules compared to the heavier iodine or lighter chlorine and fluorine. These weaker forces allow bromine molecules to transition to a liquid state at relatively low temperatures. The boiling point of bromine is only 58.8 °C (137.8 °F), further highlighting its volatility. This means bromine readily evaporates at room temperature, forming a dense, reddish-brown vapor with a pungent, irritating odor.
Intermolecular Forces and the Liquid State
The strength of intermolecular forces plays a crucial role in determining a substance's physical state. While all molecules experience London dispersion forces, the strength of these forces increases with the size and number of electrons in the molecule. Bromine, being intermediate in size and electron count amongst the halogens, exhibits a balance between these forces and thermal energy at room temperature, resulting in its liquid state. Chlorine and fluorine, with their smaller size and fewer electrons, have weaker London dispersion forces, thus remaining gases at room temperature. Conversely, iodine, with its larger size and more electrons, experiences stronger London dispersion forces, leading to its solid state.
Key Properties of Bromine
Bromine’s properties are essential to understanding its applications and handling requirements. These properties include:
- Physical State: Reddish-brown liquid at room temperature.
- Density: Relatively high density (3.12 g/cm³).
- Boiling Point: 58.8 °C (137.8 °F).
- Melting Point: -7.2 °C (19 °F).
- Solubility: Slightly soluble in water, but readily soluble in nonpolar solvents.
- Reactivity: Highly reactive, although less so than fluorine or chlorine. It readily reacts with many metals and nonmetals.
- Toxicity: Corrosive and toxic; exposure can cause severe burns and respiratory irritation. Proper safety precautions are paramount when handling bromine.
- Odor: Pungent, irritating odor.
Bromine Production: From Brine to the Element
Bromine is primarily extracted from seawater and underground brines (saltwater deposits). The production process generally involves several steps:
1. Brine Extraction and Pre-treatment
Seawater or brine is pumped to the processing facility. Depending on the source, pre-treatment might involve removing impurities or adjusting the concentration of bromide ions (Br⁻).
2. Oxidation of Bromide Ions
The bromide ions in the brine are oxidized to elemental bromine using an oxidizing agent, typically chlorine gas (Cl₂). This is a crucial step in the process, converting the bromide ions into their elemental form. The reaction can be represented as follows:
2Br⁻(aq) + Cl₂(g) → 2Cl⁻(aq) + Br₂(l)
3. Bromine Extraction and Purification
The elemental bromine formed is then extracted from the solution. This often involves techniques like stripping with air or steam, followed by condensation and purification steps. The purified bromine is then ready for packaging and distribution.
4. Alternative Sources and Production Methods
While brine is the primary source, bromine can also be extracted from other sources, such as certain minerals. Other methods exist, but chlorine-based oxidation remains the most common and commercially viable approach.
Diverse Applications of Bromine and its Compounds
Bromine's unique properties and reactivity make it a versatile element with a wide range of applications across diverse industries:
1. Flame Retardants
Brominated flame retardants (BFRs) are extensively used to improve the fire safety of various materials, including plastics, textiles, and electronics. They work by interfering with the combustion process, hindering the spread of flames. However, concerns regarding the environmental impact of certain BFRs have led to regulations and the development of alternative flame retardant technologies.
2. Agricultural Chemicals
Bromine compounds are used as fumigants and pesticides in agriculture. Methyl bromide, for instance, was widely used as a soil fumigant, but due to its ozone-depleting potential, its use is now heavily regulated under the Montreal Protocol.
3. Water Treatment
Bromine compounds are employed in water treatment for disinfection purposes. While chlorine is more common, bromine offers certain advantages in some applications due to its properties and effectiveness.
4. Medical Applications
Certain bromine compounds have found applications in medicine, although this area is less prominent compared to other applications. Some bromine-containing drugs are used in various therapeutic applications.
5. Dyes and Photography
Bromine compounds have historical applications in the dye and photography industries. While their use has diminished in some sectors, their unique chemical properties continue to be explored.
6. Industrial Chemicals and Catalysts
Bromine and its compounds serve as crucial intermediates in the production of other industrial chemicals and catalysts. Their role in various chemical processes is significant and extends to various sectors.
Environmental and Safety Concerns Associated with Bromine
Despite its numerous applications, bromine and its compounds pose certain environmental and safety concerns:
1. Toxicity and Corrosiveness
Bromine is corrosive and toxic. Direct contact with skin or inhalation of its vapors can cause severe burns, respiratory irritation, and other health issues. Therefore, proper handling and safety measures are crucial when working with bromine or its compounds.
2. Environmental Impact
Certain bromine compounds, such as some BFRs, have been linked to environmental concerns. Their persistence, bioaccumulation, and potential toxicity to wildlife have prompted regulations and the search for environmentally friendlier alternatives.
3. Ozone Depletion Potential
Methyl bromide, a previously widely used fumigant, is a potent ozone-depleting substance. Its use is now strictly regulated under international agreements like the Montreal Protocol.
4. Bioaccumulation and Biomagnification
Some bromine compounds can bioaccumulate in organisms and biomagnify in the food chain, potentially leading to harmful effects on higher trophic levels. This underscores the need for careful management and responsible use of bromine-containing compounds.
Future Prospects for Bromine and its Applications
Despite the environmental and safety concerns associated with certain bromine compounds, bromine continues to be a valuable element with potential for further development. Research is focused on developing environmentally friendly bromine-based compounds and applications, minimizing their environmental impact.
Sustainable Alternatives and Innovations
The search for sustainable alternatives to traditional bromine-based applications is ongoing. This includes the development of less persistent and less toxic compounds and the exploration of alternative flame retardants and pesticides.
Expanding Applications
Research and development continue to explore new and innovative applications for bromine and its compounds, further expanding its use in various industrial and technological fields.
Conclusion: A Versatile but Carefully Managed Element
Bromine, the only liquid halogen at room temperature, is a fascinating element with a remarkable range of properties and applications. From its crucial role in flame retardants and agricultural chemicals to its use in various industrial processes, bromine plays a significant role in modern society. However, understanding and addressing the environmental and safety concerns associated with certain bromine compounds is crucial. The future of bromine hinges on developing sustainable alternatives and innovating applications that minimize its environmental impact while maximizing its benefits. Careful management and responsible use will ensure that this versatile element continues to play a valuable role while safeguarding human health and the environment.
Latest Posts
Latest Posts
-
Chemical Equations Must Be Balanced To Satisfy The
Mar 31, 2025
-
Melting Of Butter Is A Physical Change
Mar 31, 2025
-
Why Is Dna Replication Called A Semi Conservative Process
Mar 31, 2025
-
Osmosis Involves The Movement Of What Substance
Mar 31, 2025
-
Is 1 3 An Irrational Number
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Halogen That Is Liquid At Room Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
