Chemical Equations Must Be Balanced To Satisfy The
News Leon
Mar 31, 2025 · 6 min read
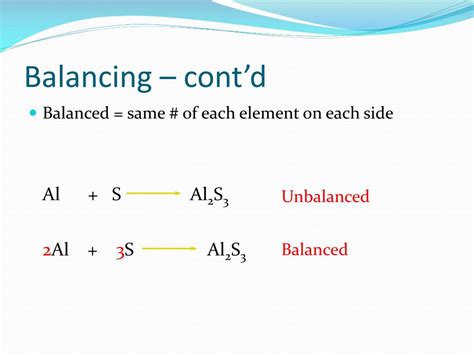
Table of Contents
Chemical Equations Must Be Balanced to Satisfy the Law of Conservation of Mass
Chemical equations are symbolic representations of chemical reactions. They show the reactants (starting materials) transforming into products (resulting substances). However, a crucial aspect of a chemical equation is that it must be balanced. This isn't just a matter of mathematical neatness; it's a fundamental requirement dictated by the Law of Conservation of Mass. This law states that matter cannot be created or destroyed in a chemical reaction; it simply changes form. Therefore, the total mass of the reactants must equal the total mass of the products. Balancing a chemical equation ensures this principle is upheld.
Understanding the Law of Conservation of Mass
The Law of Conservation of Mass is a cornerstone of chemistry. It's based on the understanding that atoms are neither gained nor lost during a chemical reaction. Atoms rearrange themselves to form new molecules, but the total number of each type of atom remains constant. This means that if you start with a certain number of atoms of each element, you must end up with the same number of atoms of each element in the products, even though they're arranged differently.
Implications for Chemical Equations
This principle has profound implications for how we write and interpret chemical equations. An unbalanced chemical equation implies that atoms are being created or destroyed, which violates the Law of Conservation of Mass. Consider this unbalanced equation:
H₂ + O₂ → H₂O
This equation suggests that two hydrogen atoms and two oxygen atoms react to produce one molecule of water (H₂O), which contains two hydrogen atoms and only one oxygen atom. Where did the other oxygen atom go? The equation, in its unbalanced state, doesn't provide a valid explanation and therefore is incorrect.
To rectify this, we must balance the equation. This involves adjusting the coefficients (the numbers in front of the chemical formulas) to ensure that the number of atoms of each element is the same on both sides of the equation.
Balancing Chemical Equations: A Step-by-Step Guide
Balancing chemical equations is a crucial skill in chemistry. While there's no single, universally foolproof method, the following steps provide a systematic approach:
1. Write the Unbalanced Equation
First, you need to write the correct chemical formulas for all the reactants and products involved in the reaction. For instance, the reaction between hydrogen and oxygen to produce water is initially written as:
H₂ + O₂ → H₂O
2. Identify the Atoms Involved
List the atoms present on both the reactant and product sides of the equation. In this example, we have hydrogen (H) and oxygen (O).
3. Count the Atoms
Count the number of atoms of each element on both the reactant and product sides. In our unbalanced equation:
- Reactants: 2 hydrogen atoms, 2 oxygen atoms
- Products: 2 hydrogen atoms, 1 oxygen atom
4. Balance the Equation
Start by balancing the element that appears in the most complex molecule. Often, it is helpful to start with metals, then nonmetals, and finally hydrogen and oxygen. In our example, oxygen appears in a more complex molecule (O₂) on the reactant side. To balance the oxygen atoms, we add a coefficient of 2 in front of the H₂O molecule on the product side:
H₂ + O₂ → 2H₂O
Now we have:
- Reactants: 2 hydrogen atoms, 2 oxygen atoms
- Products: 4 hydrogen atoms, 2 oxygen atoms
Notice that now the hydrogen atoms are unbalanced. To balance the hydrogen atoms, we need to add a coefficient of 2 in front of the H₂ molecule on the reactant side:
2H₂ + O₂ → 2H₂O
Now the equation is balanced:
- Reactants: 4 hydrogen atoms, 2 oxygen atoms
- Products: 4 hydrogen atoms, 2 oxygen atoms
The number of atoms of each element is equal on both sides, satisfying the Law of Conservation of Mass.
5. Check Your Work
Always check your balanced equation to ensure that the number of atoms of each element is equal on both sides. This final verification step is crucial to ensure accuracy.
Advanced Techniques for Balancing Chemical Equations
For more complex reactions, simple inspection may not suffice. Here are some more advanced techniques:
The Algebraic Method
For complex reactions involving many elements, using algebra can help systematically balance the equation. You assign variables to the coefficients, write equations based on the atom count for each element, and solve the system of equations to find the coefficients.
The Half-Reaction Method (for Redox Reactions)
Redox reactions, which involve electron transfer, often require a more sophisticated balancing approach. The half-reaction method breaks the overall reaction into two half-reactions (oxidation and reduction) that are balanced separately and then combined.
Importance of Balanced Chemical Equations
Beyond satisfying the Law of Conservation of Mass, balanced chemical equations provide several crucial benefits:
-
Accurate Stoichiometry: Balanced equations are essential for performing stoichiometric calculations. Stoichiometry allows us to determine the quantitative relationships between reactants and products, including the amount of reactants needed to produce a specific amount of product or vice versa. This is critical in industrial processes, chemical synthesis, and many other applications.
-
Predicting Reaction Yields: Balanced equations help predict the theoretical yield of a reaction, which is the maximum amount of product that can be formed based on the amount of limiting reactant. This allows chemists and engineers to optimize reaction conditions for maximum efficiency.
-
Understanding Reaction Mechanisms: While balancing an equation doesn't reveal the reaction mechanism (the step-by-step process by which the reaction occurs), it provides a framework for understanding the overall transformation. This knowledge can inform the development of new catalysts or reaction conditions.
-
Environmental Considerations: In environmental chemistry, balanced equations are crucial for understanding pollutant formation and remediation. They help quantify the amount of pollutants produced and the effectiveness of different cleanup strategies.
Common Mistakes to Avoid When Balancing Equations
Balancing chemical equations can be tricky. Here are some common mistakes to watch out for:
-
Changing Subscripts: Never alter the subscripts within a chemical formula. Subscripts define the chemical composition of a molecule. Changing them changes the identity of the substance entirely. Only change the coefficients.
-
Forgetting to Balance All Elements: Ensure you check each element present in the equation to verify that it’s balanced on both sides.
-
Rushing the Process: Take your time, work methodically, and double-check your work before considering the equation balanced.
Conclusion
Balancing chemical equations is not merely a mathematical exercise; it’s a fundamental principle rooted in the Law of Conservation of Mass. This law dictates that matter cannot be created or destroyed in a chemical reaction; only transformed. By balancing equations, we ensure that our representation of chemical reactions accurately reflects this fundamental principle, enabling accurate predictions, stoichiometric calculations, and a deeper understanding of chemical processes. Mastering the art of balancing chemical equations is essential for any aspiring chemist, scientist, or engineer. The ability to accurately and efficiently balance even complex equations forms the basis for further exploration and understanding in the vast and intricate world of chemical reactions. This skill allows for accurate modeling and manipulation of reactions in numerous applications, from industrial processes to environmental management. So, whether you're a student learning the basics or a seasoned professional working on cutting-edge research, remember: a balanced equation is not just correct, it's essential.
Latest Posts
Latest Posts
-
Draw And Label One Complete Cell Cycle
Apr 02, 2025
-
A Physical Combination Of Two Or More Substances
Apr 02, 2025
-
What Is The Difference Between A Primary And Secondary Consumer
Apr 02, 2025
-
Which One Of The Following Is An Igneous Rock
Apr 02, 2025
-
Which Is Greater 2 3 Or 3 5
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Chemical Equations Must Be Balanced To Satisfy The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
