Osmosis Involves The Movement Of What Substance
News Leon
Mar 31, 2025 · 6 min read
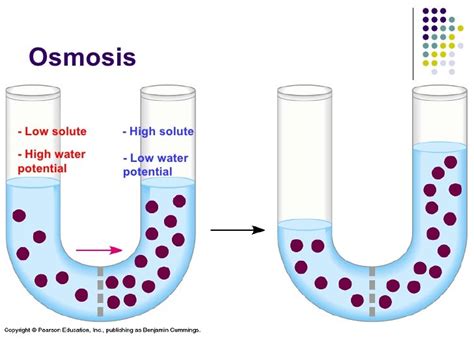
Table of Contents
Osmosis: The Movement of Water Across Membranes
Osmosis, a fundamental process in biology and chemistry, is defined as the movement of water molecules across a selectively permeable membrane from a region of high water concentration to a region of low water concentration. This movement continues until equilibrium is reached, meaning the water concentration is equal on both sides of the membrane. Understanding this simple definition, however, requires delving into the specifics of water's behavior, membrane properties, and the implications of osmosis for living organisms. This article will explore osmosis in detail, covering its mechanism, factors influencing it, and its vital role in biological systems.
What is a Selectively Permeable Membrane?
Before diving into the intricacies of osmosis, it's crucial to understand the role of the selectively permeable membrane. This membrane, often composed of a lipid bilayer with embedded proteins, acts as a gatekeeper, controlling the passage of substances. It's "selectively permeable" because it allows some molecules to pass through freely while restricting the movement of others. Water, being a small, polar molecule, can often pass through these membranes relatively easily, although the rate can vary depending on membrane composition and structure. Larger molecules, charged ions, and other substances are generally prevented from crossing freely, resulting in a difference in concentration across the membrane. This difference in concentration is what drives the process of osmosis.
The Driving Force: Water Potential
The movement of water in osmosis isn't random; it's driven by a difference in water potential. Water potential is a measure of the potential energy of water, reflecting its tendency to move from one area to another. It's influenced by several factors, including:
1. Solute Potential (Ψs):
Solute potential is the effect of dissolved solutes on water potential. The presence of solutes lowers the water potential because the solutes reduce the free movement of water molecules. A higher concentration of solutes means a lower (more negative) solute potential. Pure water has a solute potential of zero.
2. Pressure Potential (Ψp):
Pressure potential arises from physical pressure exerted on the water. Positive pressure potential (turgor pressure) occurs when water is forced against a rigid structure like a cell wall, increasing the water potential. Negative pressure potential occurs when tension is applied to the water, such as in the xylem of plants, reducing the water potential.
Total Water Potential (Ψ):
The total water potential is the sum of the solute potential and the pressure potential: Ψ = Ψs + Ψp. Water moves from an area of higher water potential to an area of lower water potential.
Osmosis in Action: Hypotonic, Hypertonic, and Isotonic Solutions
To understand how osmosis works in practice, let's consider different types of solutions:
1. Hypotonic Solution:
A hypotonic solution has a lower solute concentration (and therefore a higher water potential) than the solution it's compared to. When a cell is placed in a hypotonic solution, water moves into the cell because the water potential inside the cell is lower. This can cause the cell to swell and potentially burst (lyse) in animal cells, which lack a rigid cell wall. In plant cells, the cell wall prevents bursting, resulting in turgor pressure, which is essential for maintaining plant cell shape and rigidity.
2. Hypertonic Solution:
A hypertonic solution has a higher solute concentration (and therefore a lower water potential) than the solution it's compared to. When a cell is placed in a hypertonic solution, water moves out of the cell due to the higher water potential inside. This causes the cell to shrink and plasmolyze, particularly visible in plant cells where the plasma membrane pulls away from the cell wall. Animal cells also shrink in hypertonic solutions, a process called crenation.
3. Isotonic Solution:
An isotonic solution has the same solute concentration (and therefore the same water potential) as the solution it's compared to. In an isotonic solution, there's no net movement of water across the membrane; the water movement into and out of the cell is equal. The cell maintains its shape and size.
The Importance of Osmosis in Biological Systems
Osmosis plays a critical role in numerous biological processes:
1. Water Uptake in Plants:
Plants absorb water from the soil through their roots via osmosis. The soil water typically has a higher water potential than the cells in the root hairs, driving water into the plant. This water is then transported throughout the plant via the xylem, contributing to cell turgor and maintaining the plant's structure.
2. Water Regulation in Animals:
Animals maintain water balance through osmoregulation, a process involving osmosis. Kidneys play a vital role in this process, controlling the concentration of solutes in the blood and excreting excess water or retaining water as needed to maintain osmotic balance.
3. Cell Volume Regulation:
Osmosis ensures that cells maintain a suitable internal environment. Cells need to regulate their volume to prevent damage from swelling or shrinking. This is crucial for cell function and survival.
4. Nutrient Absorption:
Osmosis can indirectly influence nutrient absorption. The movement of water into cells can facilitate the uptake of dissolved nutrients, as water flow creates a concentration gradient that helps transport nutrients into cells.
5. Waste Removal:
Osmosis contributes to waste removal by facilitating the movement of waste products out of cells and into the circulatory system for excretion.
Factors Affecting the Rate of Osmosis
Several factors influence the rate at which osmosis occurs:
- Concentration Gradient: A steeper concentration gradient (larger difference in water potential) leads to a faster rate of osmosis.
- Temperature: Higher temperatures generally increase the rate of osmosis as water molecules have more kinetic energy.
- Membrane Permeability: A more permeable membrane allows for a faster rate of osmosis. The size and type of pores within the membrane influence permeability.
- Surface Area: A larger surface area of the membrane facilitates a faster rate of osmosis.
- Pressure: Applying pressure can influence the rate and direction of osmosis.
Osmosis vs. Diffusion
While both osmosis and diffusion involve the movement of substances from a region of high concentration to a region of low concentration, there's a key difference:
- Diffusion refers to the movement of any substance (solids, liquids, gases) across a membrane or within a solution.
- Osmosis specifically refers to the movement of water across a selectively permeable membrane.
Essentially, osmosis is a specialized type of diffusion involving water.
Reverse Osmosis: A Technological Application
Reverse osmosis is a technological application of osmosis that uses external pressure to force water across a semipermeable membrane against its natural concentration gradient. This process is used for water purification, removing dissolved impurities and creating potable water from sources like saltwater.
Conclusion
Osmosis is a critical process fundamental to life, playing a central role in water balance, nutrient transport, and cell function in both plants and animals. Understanding the principles of osmosis, including water potential, membrane permeability, and the influence of different solution types, is crucial for grasping numerous biological phenomena. The process of osmosis is not just a passive movement of water; it is a dynamic process driven by intricate biological mechanisms and plays a crucial role in maintaining the delicate balance necessary for life. From the smallest single-celled organism to the largest redwood tree, the simple yet powerful process of osmosis underlies many of life's essential functions.
Latest Posts
Latest Posts
-
Which Is Greater 2 3 Or 3 5
Apr 02, 2025
-
Find The Area Of A Shaded Triangle
Apr 02, 2025
-
Which Of The Following Would Decrease Glomerular Filtration Rate
Apr 02, 2025
-
The Slope Of Speed Time Graph Indicates
Apr 02, 2025
-
What Is The Approximate Size Of A Nucleus
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Osmosis Involves The Movement Of What Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
