What Is The Mass Number For Silver
News Leon
Mar 23, 2025 · 5 min read
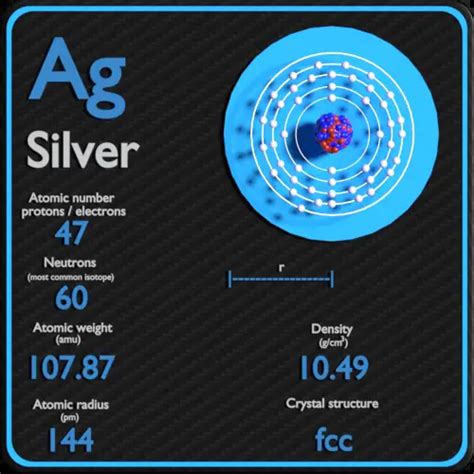
Table of Contents
What is the Mass Number for Silver? Understanding Isotopes and Atomic Mass
Silver, a lustrous and precious metal known for its conductivity and use in jewelry and electronics, presents a fascinating case study in atomic structure. Unlike elements with a single, readily defined mass number, silver possesses a range of isotopes, each contributing to its overall average atomic mass. Understanding the concept of mass number and its application to silver requires delving into the world of isotopes and their relative abundances.
What is Mass Number?
The mass number of an atom represents the total number of protons and neutrons found in its nucleus. Protons and neutrons are collectively known as nucleons. It's crucial to differentiate the mass number from the atomic number. The atomic number, denoted by Z, solely represents the number of protons in an atom's nucleus – a defining characteristic that determines the element's identity. Silver, for example, always has 47 protons (Z = 47), making it silver regardless of its mass number.
The number of neutrons, often denoted by N, can vary within a given element, leading to the existence of isotopes. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. Therefore, isotopes have the same atomic number but different mass numbers.
Silver's Isotopes: A Closer Look
Silver exists naturally as a mixture of two stable isotopes:
-
Silver-107 (¹⁰⁷Ag): This isotope accounts for approximately 51.84% of naturally occurring silver. Its mass number is 107, meaning it has 47 protons (its atomic number) and 60 neutrons (107 - 47 = 60).
-
Silver-109 (¹⁰⁹Ag): Making up the remaining 48.16%, silver-109 also boasts 47 protons but has 62 neutrons (109 - 47 = 62).
It's crucial to remember that the mass number is a whole number; it represents the sum of protons and neutrons, which are discrete particles.
The Concept of Atomic Mass
While the mass number for each silver isotope is definitive (107 and 109), the atomic mass (or atomic weight) of silver is different. The atomic mass is a weighted average of the masses of all naturally occurring isotopes of an element. The weighting is based on the relative abundance of each isotope. This is why the atomic mass you'll find on the periodic table for silver is not a whole number.
To calculate the atomic mass of silver, we use the following formula:
Atomic Mass = (fractional abundance of ¹⁰⁷Ag × mass of ¹⁰⁷Ag) + (fractional abundance of ¹⁰⁹Ag × mass of ¹⁰⁹Ag)
Using the approximate values for isotopic abundances and masses:
Atomic Mass ≈ (0.5184 × 106.90509 u) + (0.4816 × 108.90476 u) ≈ 107.8682 u
Where 'u' represents the atomic mass unit, approximately the mass of a single proton or neutron. The value 107.8682 u is very close to the atomic mass of silver found on the periodic table, demonstrating the principle of weighted average.
Why Isn't the Atomic Mass a Whole Number?
The atomic mass of silver, and indeed most elements, is not a whole number due to the presence of multiple isotopes with different masses. The weighted average of these isotopic masses results in a decimal value reflecting the relative abundances of each isotope.
This fractional atomic mass reflects the reality of elemental composition in nature. It’s not an indication of a fractional number of protons or neutrons within a single atom but rather a macroscopic average representing the composition of a large number of silver atoms.
Applications of Silver Isotopes
Understanding the isotopic composition of silver has practical implications in various fields:
1. Archaeology and Dating Techniques:
The relative abundances of silver isotopes can provide insights into the origins and age of ancient artifacts made from silver. Slight variations in isotopic ratios can pinpoint geographic locations of silver sources.
2. Industrial Processes:
Controlling the isotopic composition of silver used in certain industrial processes, such as in photography or electronics, can affect the performance and characteristics of the final product.
3. Medical and Scientific Research:
Silver isotopes, particularly radioactive isotopes, find application in medical imaging and tracing processes within the body.
Silver's Role in Different Fields
Silver’s unique properties contribute to its widespread use in various industries:
1. Jewelry and Ornamentation:
Silver's lustrous appearance and malleability have made it a highly sought-after material for jewelry and decorative objects for centuries.
2. Electronics:
Silver's exceptional electrical conductivity makes it invaluable in electronics, particularly in high-frequency circuits and conductive inks.
3. Photography:
Historically, silver compounds played a crucial role in photographic processes, although digital photography has largely replaced traditional methods.
4. Catalysis:
Silver's catalytic properties are used in various chemical reactions, including the oxidation of ethylene to ethylene oxide.
Beyond Silver: Isotopes and the Periodic Table
The concept of isotopes and their influence on atomic mass is not unique to silver. Many elements exist as mixtures of isotopes, each contributing to the overall average atomic mass reported on the periodic table. Understanding this nuanced aspect of atomic structure is critical for comprehending the behavior of elements and their applications in diverse fields. The periodic table, while presenting a simplified view of elemental properties, reflects the underlying complexity of isotopic distributions.
Conclusion: A Comprehensive Understanding
In summary, while silver doesn't possess a single mass number, it exists as a mixture of two stable isotopes, ¹⁰⁷Ag and ¹⁰⁹Ag, with mass numbers 107 and 109 respectively. The atomic mass of silver (approximately 107.8682 u), as listed on the periodic table, is a weighted average reflecting the relative abundances of these isotopes. This understanding is fundamental in various scientific and industrial applications, emphasizing the importance of isotopic composition in the properties and behavior of elements. The concepts discussed here highlight the interconnectedness of atomic structure, isotopic variations, and the macroscopic properties of elements, underscoring the intricate details within the seemingly simple representation of elements on the periodic table. The detailed exploration of silver's isotopes allows for a clearer understanding of the broader concepts of mass number, atomic mass, and the importance of isotopic analysis across scientific disciplines.
Latest Posts
Latest Posts
-
Select The Correct Iupac Name For The Following Compound
Mar 25, 2025
-
How Many Atoms Are In A Single Molecule Of Water
Mar 25, 2025
-
Electric Field Infinite Line Of Charge
Mar 25, 2025
-
A Pure Substance Containing Only One Kind Of
Mar 25, 2025
-
A Species Is A Group Of Organisms That
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Mass Number For Silver . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
