Select The Correct Iupac Name For The Following Compound
News Leon
Mar 25, 2025 · 6 min read
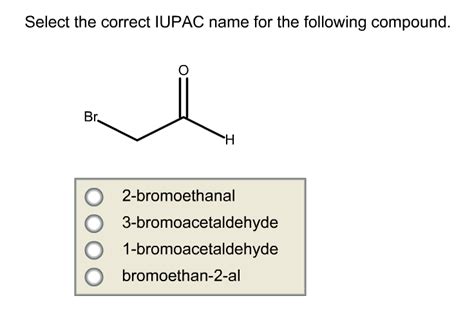
Table of Contents
Selecting the Correct IUPAC Name for Organic Compounds: A Comprehensive Guide
Naming organic compounds might seem daunting at first, but mastering the International Union of Pure and Applied Chemistry (IUPAC) nomenclature system is crucial for clear communication in organic chemistry. This comprehensive guide will walk you through the process, tackling various complexities and providing examples to solidify your understanding. We'll explore the intricacies of selecting the correct IUPAC name for a given compound, covering alkanes, alkenes, alkynes, alcohols, ketones, aldehydes, carboxylic acids, and more.
Understanding the Fundamentals of IUPAC Nomenclature
The IUPAC system follows a set of hierarchical rules to ensure that every organic compound has a unique, unambiguous name. These rules prioritize:
- Identifying the longest carbon chain: This forms the base name of the compound.
- Numbering the carbon chain: This is done to assign locants (numbers) to substituents and functional groups.
- Naming substituents and functional groups: Each branch or functional group is named and its position on the parent chain is indicated.
- Arranging substituents alphabetically: This dictates the order in which substituents are listed in the name.
- Using prefixes and suffixes: These indicate the type and number of substituents and the presence of functional groups.
Alkanes: The Foundation of Organic Nomenclature
Alkanes are hydrocarbons containing only single bonds. Their IUPAC names follow a simple pattern:
- Meth- (1 carbon), Eth- (2 carbons), Prop- (3 carbons), But- (4 carbons), Pent- (5 carbons), Hex- (6 carbons), Hept- (7 carbons), Oct- (8 carbons), Non- (9 carbons), Dec- (10 carbons), and so on.
The suffix "-ane" indicates a saturated hydrocarbon (all single bonds).
Example: A straight chain alkane with 5 carbons is called pentane.
Incorporating Branches and Substituents
When branches (alkyl groups) are present, we need to:
- Identify the longest continuous carbon chain: This forms the parent alkane.
- Number the carbon atoms in the parent chain: Start from the end closest to the first branch.
- Name the branches (alkyl groups): Methyl (CH3), ethyl (CH2CH3), propyl (CH2CH2CH3), etc.
- Indicate the position of each branch using a number (locant): This number precedes the name of the alkyl group.
- List the branches alphabetically: Ignore prefixes like "di-", "tri-", etc., when alphabetizing.
Example: Consider a compound with a propane chain and a methyl group attached to the second carbon. The correct IUPAC name is 2-methylpropane.
Dealing with Multiple Substituents
If there are multiple substituents, we:
- Number the parent chain to give the lowest possible set of locants.
- List the substituents alphabetically.
- Use prefixes (di-, tri-, tetra-, etc.) to indicate the number of identical substituents.
Example: A compound with two methyl groups on the second carbon of butane would be named 2,2-dimethylbutane. A compound with a methyl group on carbon 2 and an ethyl group on carbon 3 would be 3-ethyl-2-methylpentane (ethyl comes before methyl alphabetically).
Unsaturated Hydrocarbons: Alkenes and Alkynes
Alkenes contain at least one carbon-carbon double bond, and alkynes contain at least one carbon-carbon triple bond. The naming conventions are similar to alkanes, but with crucial differences:
- Suffix: "-ene" for alkenes and "-yne" for alkynes.
- Locant: The double or triple bond must be indicated by its lowest possible number.
Example: CH2=CHCH3 is prop-1-ene. CH≡CCH2CH3 is but-1-yne.
Cyclic Compounds: Cycloalkanes and Cycloalkenes
Cyclic compounds are named using the prefix "cyclo-" followed by the name of the corresponding alkane or alkene.
- Cycloalkanes: These have only single bonds within the ring.
- Cycloalkenes: These have at least one double bond within the ring.
Example: A six-membered ring with only single bonds is called cyclohexane. A six-membered ring with one double bond is named cyclohexene. The position of the double bond is indicated if necessary.
Functional Groups: Adding Complexity
Functional groups are specific atoms or groups of atoms that impart characteristic chemical properties to organic molecules. They significantly influence the naming process. The IUPAC system uses suffixes and prefixes to designate functional groups:
-
Alcohols (-OH): Suffix "-ol". The position of the hydroxyl group is indicated with a number. Example: CH3CH2OH is ethanol.
-
Ketones (C=O): Suffix "-one". The position of the carbonyl group is indicated. Example: CH3COCH3 is propan-2-one (or acetone).
-
Aldehydes (CHO): Suffix "-al". Example: CH3CHO is ethanal (or acetaldehyde).
-
Carboxylic acids (COOH): Suffix "-oic acid". Example: CH3COOH is ethanoic acid (or acetic acid).
-
Amines (-NH2): Prefix "amino-". Example: CH3CH(NH2)COOH is 2-aminopropanoic acid.
-
Ethers (R-O-R'): The alkyl groups are listed alphabetically, followed by the word "ether". Example: CH3OCH2CH3 is ethyl methyl ether.
-
Halogenated compounds (F, Cl, Br, I): Prefixes fluoro-, chloro-, bromo-, iodo- are used, followed by the number indicating the position. Example: CH3CHClCH3 is 2-chloropropane.
Prioritizing Functional Groups
When a molecule contains multiple functional groups, a priority order determines which group dictates the suffix and which are treated as prefixes. Carboxylic acids generally have the highest priority, followed by aldehydes, ketones, alcohols, etc. The specific hierarchy should be consulted for complex cases.
Stereochemistry: Chirality and Isomerism
The IUPAC system also accounts for stereochemistry, including chirality (handedness) and isomerism (different arrangements of atoms). Designations like (R) and (S) indicate the absolute configuration of chiral centers. cis-/trans- or E/Z- notation describes the relative arrangement of substituents around double bonds. These details add layers of complexity but are essential for complete and accurate naming.
Practical Examples and Troubleshooting
Let’s analyze a few more complex examples to cement our understanding:
Example 1: Consider the compound with a cyclohexane ring, a methyl group at position 1, and an ethyl group at position 4. The name would be 1-methyl-4-ethylcyclohexane.
Example 2: A compound with a long chain containing a double bond and a hydroxyl group: The higher priority functional group (alcohol -OH) determines the suffix "-ol". Numbering should prioritize the hydroxyl group location while still giving the double bond the lowest number possible.
Example 3: A molecule with both a carboxylic acid and a ketone group. The carboxylic acid will determine the suffix "-oic acid," and the ketone will be indicated using the prefix "oxo-". Numbering would start from the carboxyl carbon.
Navigating these complex examples highlights the importance of systematic and step-by-step application of the IUPAC rules. Remember to meticulously identify the longest chain, number it correctly, alphabetize substituents, and prioritize functional groups.
Advanced Considerations and Resources
This guide covers the fundamental principles of IUPAC nomenclature. More advanced topics, such as heterocyclic compounds, complex ring systems, and specific naming conventions for different classes of organic molecules, require deeper study. Consult comprehensive organic chemistry textbooks and dedicated IUPAC resources for detailed information and specific cases. Practice is key to mastering IUPAC nomenclature. Working through numerous examples, both simple and complex, will significantly improve your ability to correctly name organic compounds.
Remember, clear and unambiguous naming of compounds is critical for accurate scientific communication. Mastering IUPAC nomenclature is a rewarding skill that will significantly benefit your understanding and application of organic chemistry.
Latest Posts
Latest Posts
-
Where Does The Krebs Cycle Take Place In The Mitochondria
Mar 26, 2025
-
Correctly Label The Following Parts Of The Testis
Mar 26, 2025
-
Which Of The Following Equations Is True
Mar 26, 2025
-
The Molar Mass Of Cuso4 5h2o Is 249
Mar 26, 2025
-
Enzymes Belong To Which Group Of Macromolecules
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Select The Correct Iupac Name For The Following Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
