How Many Atoms Are In A Single Molecule Of Water
News Leon
Mar 25, 2025 · 6 min read
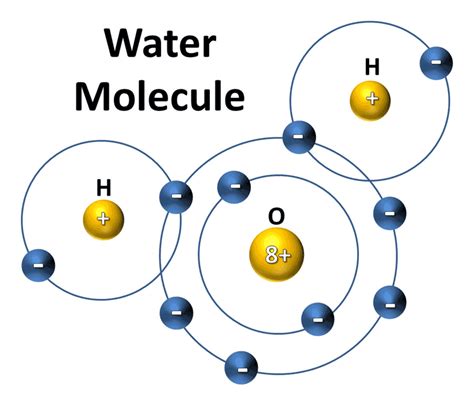
Table of Contents
How Many Atoms Are in a Single Molecule of Water? A Deep Dive into Atomic Composition
Water. We drink it, bathe in it, and it covers most of our planet. But have you ever stopped to think about the incredibly tiny components that make up even a single drop of this essential substance? This article delves into the fascinating world of atoms and molecules, specifically exploring the atomic composition of a water molecule and the broader implications of understanding its structure.
Understanding Atoms and Molecules
Before we dive into the specifics of water, let's establish a foundational understanding of atoms and molecules.
Atoms: The Fundamental Building Blocks
Atoms are the smallest units of matter that retain the chemical properties of an element. They consist of a dense central nucleus containing protons (positively charged) and neutrons (neutrally charged), surrounded by a cloud of orbiting electrons (negatively charged). The number of protons in an atom's nucleus determines its atomic number and identifies the element. For instance, an atom with one proton is hydrogen, while an atom with eight protons is oxygen.
Molecules: Atoms Bonding Together
When atoms combine, they form molecules. This combination happens through chemical bonds, which are essentially the attractive forces holding atoms together. These bonds are formed through the sharing or transfer of electrons between atoms. The type and number of atoms in a molecule determine its properties and behavior.
The Water Molecule: H₂O
Now, let's focus on the star of our show: water (H₂O). The chemical formula H₂O tells us everything we need to know about its composition:
- H: Represents the element hydrogen.
- ₂: Indicates there are two atoms of hydrogen in each water molecule.
- O: Represents the element oxygen.
- Therefore, a single water molecule contains two hydrogen atoms and one oxygen atom.
This seemingly simple composition belies the incredible properties of water, making it essential for life as we know it.
The Covalent Bonds in H₂O
The atoms in a water molecule are held together by covalent bonds. In a covalent bond, atoms share electrons to achieve a more stable electron configuration. Oxygen is highly electronegative, meaning it attracts electrons strongly. It shares electrons with each of the two hydrogen atoms, forming a stable molecule.
Polarity of the Water Molecule
The unequal sharing of electrons between oxygen and hydrogen in water results in a polar molecule. This means that one end of the molecule (the oxygen end) carries a slightly negative charge (δ-), while the other end (the hydrogen ends) carries a slightly positive charge (δ+). This polarity is crucial for many of water's unique properties, such as its ability to act as a solvent and its high surface tension.
The Significance of the Number of Atoms
The precise number of atoms in a water molecule – two hydrogen and one oxygen – dictates its physical and chemical characteristics. Even a slight change in this composition would result in a drastically different substance. For example, hydrogen peroxide (H₂O₂) has an extra oxygen atom, giving it entirely different properties and making it a potent oxidizer.
Exploring the Vastness: From Molecules to Moles
While understanding the atomic composition of a single water molecule is crucial, it's equally important to consider the scale at which we usually interact with water. We rarely deal with individual molecules; instead, we deal with massive quantities. This is where the concept of a mole comes into play.
The Mole: A Chemist's Dozen
A mole (mol) is a unit in chemistry that represents a specific number of particles, specifically Avogadro's number, which is approximately 6.022 x 10²³. This means one mole of water contains 6.022 x 10²³ water molecules.
Calculating Atoms in a Mole of Water
Since each water molecule has three atoms (two hydrogen and one oxygen), one mole of water contains:
- 2 x (6.022 x 10²³) hydrogen atoms = 1.204 x 10²⁴ hydrogen atoms
- 1 x (6.022 x 10²³) oxygen atoms = 6.022 x 10²³ oxygen atoms
This illustrates the vast number of atoms present in even a small amount of water.
The Importance of Understanding Water's Atomic Structure
Understanding the precise atomic composition of water is fundamental to numerous fields:
- Chemistry: It's crucial for understanding chemical reactions involving water, its role as a solvent, and its unique properties.
- Biology: Water's unique properties are essential for life. Its polarity facilitates crucial biological processes.
- Environmental Science: The understanding of water's atomic structure is crucial for studying water cycles, pollution, and water treatment.
- Physics: Water's properties, dictated by its atomic structure, are studied in various physics branches, including thermodynamics and fluid mechanics.
- Materials Science: Water's interaction with other materials is heavily influenced by its atomic structure.
Beyond Water: Expanding the Knowledge
The principles discussed here regarding the atomic composition of water apply to all molecules. Every substance, whether a simple gas or a complex biological molecule, is built from specific combinations of atoms. Understanding these combinations is key to understanding the properties and behavior of all matter.
Frequently Asked Questions (FAQs)
Q: Can a water molecule exist without all three atoms?
A: No. A water molecule requires precisely two hydrogen atoms and one oxygen atom. Removing or adding an atom fundamentally alters the substance.
Q: What if a water molecule loses one of its hydrogen atoms?
A: Losing a hydrogen atom would result in a hydroxide ion (OH⁻), a highly reactive species with significantly different properties than water.
Q: How can we visualize water molecules?
A: While we can't see individual molecules with the naked eye, advanced microscopic techniques like electron microscopy and various imaging methods allow scientists to visualize molecular structures and behaviors.
Q: Are all water molecules exactly the same?
A: While the basic composition (H₂O) is the same, slight variations can occur due to isotopes. Isotopes are atoms of the same element with different numbers of neutrons. For example, some water molecules might contain deuterium (²H), a heavier isotope of hydrogen.
Q: How does understanding the number of atoms in water relate to larger-scale phenomena?
A: Understanding the atomic composition of water is crucial to comprehending its macroscopic properties like boiling point, freezing point, density, and its role as a universal solvent. These properties influence climate patterns, biological functions, and numerous industrial processes.
Q: What are some real-world applications of this knowledge?
A: The understanding of water's structure is vital for water purification technologies, the development of new materials with water-resistant properties, and in medical fields for developing solutions and treatments that interact with the body's water content.
Conclusion
The seemingly simple question of how many atoms are in a water molecule opens a gateway to a vast and complex world of atomic structure, molecular interactions, and the remarkable properties of matter. By understanding the precise composition of water (two hydrogen atoms and one oxygen atom), we unlock insights into its essential role in our world, from sustaining life to shaping our environment. This knowledge forms the bedrock for advancements in numerous scientific and technological fields, highlighting the significant impact of fundamental scientific principles on our daily lives. From the smallest molecule to the largest ocean, the understanding of atomic composition remains a cornerstone of scientific progress and exploration.
Latest Posts
Latest Posts
-
Atrioventricular Valves Prevent Backflow Into The
Mar 26, 2025
-
The Oxygen Released During Photosynthesis Comes From Where
Mar 26, 2025
-
What Percent Is 70 Of 280
Mar 26, 2025
-
Where Does The Krebs Cycle Take Place In The Mitochondria
Mar 26, 2025
-
Correctly Label The Following Parts Of The Testis
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In A Single Molecule Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
