What Is The Final Electron Acceptor In The Etc
News Leon
Mar 28, 2025 · 7 min read
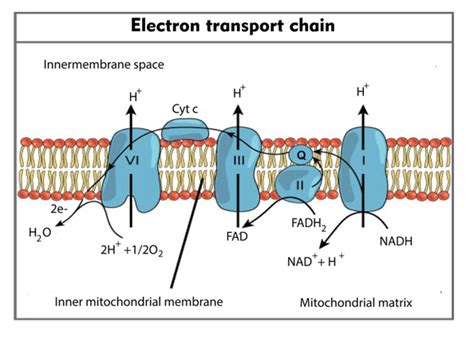
Table of Contents
What is the Final Electron Acceptor in the ETC? Understanding Oxidative Phosphorylation
The electron transport chain (ETC), also known as the respiratory chain, is a series of protein complexes embedded in the inner mitochondrial membrane of eukaryotes and the plasma membrane of prokaryotes. Its primary function is to facilitate the transfer of electrons from electron donors (like NADH and FADH2) to a final electron acceptor, ultimately driving the synthesis of ATP, the cell's energy currency. Understanding the final electron acceptor is crucial to comprehending the entire process of oxidative phosphorylation and cellular respiration. This article will delve deep into the intricacies of the ETC, focusing specifically on the identity and role of the final electron acceptor.
The Electron Transport Chain: A Step-by-Step Overview
Before identifying the final electron acceptor, let's briefly review the steps involved in the ETC. The process begins with the entry of high-energy electrons from NADH and FADH2, which are produced during glycolysis and the citric acid cycle (Krebs cycle). These molecules act as electron carriers, donating their electrons to the ETC complexes.
Complex I: NADH Dehydrogenase
The journey starts at Complex I, also known as NADH dehydrogenase. Here, NADH donates two electrons, which are then passed down a series of electron carriers within Complex I. This electron transfer is coupled to the pumping of protons (H⁺) from the mitochondrial matrix to the intermembrane space, establishing a proton gradient.
Complex II: Succinate Dehydrogenase
Complex II, or succinate dehydrogenase, is slightly different. It's the only ETC complex directly involved in the citric acid cycle. FADH2, another electron carrier produced during the citric acid cycle, delivers its electrons to Complex II. Unlike Complex I, Complex II does not pump protons across the membrane.
Complex III: Cytochrome bc₁ Complex
The electrons from both Complex I and Complex II are passed to Complex III, also known as the cytochrome bc₁ complex. This complex contains heme groups and iron-sulfur clusters that facilitate electron transfer. Importantly, Complex III also contributes to the proton gradient by pumping protons into the intermembrane space.
Complex IV: Cytochrome c Oxidase
Finally, the electrons are transferred to Complex IV, cytochrome c oxidase. This complex is the terminal oxidase of the ETC and plays a vital role in the final step of electron transfer.
The Final Electron Acceptor: Oxygen's Crucial Role
The final electron acceptor in the electron transport chain is molecular oxygen (O₂). After passing through Complexes I, III, and IV, the electrons are ultimately transferred to oxygen. This is a crucial step because oxygen's high electronegativity allows it to readily accept electrons. The reduction of oxygen is a thermodynamically favorable process, driving the entire electron transport chain forward.
The Reduction of Oxygen: Formation of Water
The addition of electrons to oxygen doesn't simply create a different oxygen species; it results in the formation of water. Each oxygen molecule (O₂) accepts four electrons and four protons (H⁺) to produce two water molecules (2H₂O). This reaction is represented as:
O₂ + 4e⁻ + 4H⁺ → 2H₂O
This seemingly simple reaction is the culmination of a complex series of redox reactions within the ETC. The formation of water is essential for maintaining the flow of electrons and the generation of the proton gradient, which is necessary for ATP synthesis.
The Proton Motive Force and ATP Synthesis
The proton gradient established across the inner mitochondrial membrane by Complexes I, III, and IV is crucial for ATP synthesis. This gradient creates a proton motive force (PMF), which drives ATP synthesis via chemiosmosis. Protons flow back into the mitochondrial matrix through ATP synthase, an enzyme that utilizes the energy from the proton flow to phosphorylate ADP to ATP.
Alternative Electron Acceptors: Anaerobic Respiration
While oxygen is the most common and efficient final electron acceptor in the ETC, some organisms can utilize alternative electron acceptors under anaerobic conditions (i.e., in the absence of oxygen). This process, known as anaerobic respiration, still involves an electron transport chain, but the final electron acceptor is different. Examples of alternative electron acceptors include:
- Nitrate (NO₃⁻): Used by denitrifying bacteria, reducing nitrate to nitrite (NO₂⁻) or even nitrogen gas (N₂).
- Sulfate (SO₄²⁻): Used by sulfate-reducing bacteria, reducing sulfate to hydrogen sulfide (H₂S).
- Carbon dioxide (CO₂): Used by methanogenic archaea, reducing carbon dioxide to methane (CH₄).
- Fumarate: Used by some bacteria in anaerobic conditions, reducing fumarate to succinate.
These alternative electron acceptors have lower reduction potentials than oxygen, meaning that less energy is released during the electron transfer process. Consequently, anaerobic respiration produces less ATP than aerobic respiration.
The Importance of Oxygen as the Final Electron Acceptor
Oxygen's role as the final electron acceptor in the ETC is paramount for efficient energy production. Its high electronegativity ensures that the electron transfer process is highly exergonic (releases a large amount of energy), maximizing the energy captured for ATP synthesis. Without oxygen, the ETC would become blocked, halting the flow of electrons and significantly reducing ATP production. This explains why aerobic respiration is so much more efficient than anaerobic respiration.
Clinical Significance: Oxygen Deficiency and Disease
Disruptions in the electron transport chain, often due to oxygen deficiency (hypoxia) or mitochondrial dysfunction, can have severe consequences. Conditions like mitochondrial myopathies and various metabolic disorders are linked to impaired ETC function. These conditions can manifest as muscle weakness, fatigue, and other systemic problems.
The ETC: A Dynamic and Vital Process
The electron transport chain is a highly complex and dynamic process essential for life. The final electron acceptor, oxygen, plays a crucial role in driving the entire process, enabling efficient energy production through oxidative phosphorylation. Understanding the ETC and the role of oxygen is vital for comprehending cellular respiration and the pathogenesis of various diseases linked to mitochondrial dysfunction. Further research into the intricacies of the ETC continues to reveal new insights into its regulation and clinical significance.
Beyond the Basics: Regulation and Control of the ETC
The efficiency of the ETC isn't simply a matter of having the correct components; it's also finely regulated to match the cell's energy demands. Several mechanisms control the rate of electron transport:
-
Substrate Availability: The availability of NADH and FADH2, the electron donors, directly influences the rate of the ETC. High levels of these molecules stimulate electron flow, while low levels inhibit it.
-
Oxygen Concentration: Oxygen availability is a critical factor. Reduced oxygen levels (hypoxia) limit the final electron acceptor, slowing down the entire process.
-
Feedback Inhibition: The ATP/ADP ratio acts as a feedback mechanism. High ATP levels inhibit the ETC, while low ATP levels stimulate it. This ensures that ATP production is balanced with cellular energy demands.
-
Uncoupling Proteins: These proteins facilitate the passage of protons across the inner mitochondrial membrane without ATP synthesis. They effectively "uncouple" electron transport from ATP production, generating heat instead. This is important in brown adipose tissue for thermogenesis.
These regulatory mechanisms ensure that the ETC operates efficiently and adapts to the cell's fluctuating energy needs, preventing wasteful energy expenditure and maintaining cellular homeostasis.
Future Research and Applications: Targeting the ETC
The ETC remains a significant area of research. Understanding its regulation and function is crucial for developing therapeutic strategies for diseases linked to mitochondrial dysfunction. Current research focuses on:
-
Developing drugs targeting the ETC: This could offer potential treatments for mitochondrial diseases, cancer (by targeting cancer cell mitochondria), and other conditions.
-
Exploring alternative electron acceptors: Research is ongoing to develop biotechnological applications utilizing alternative electron acceptors for bioremediation and energy production.
-
Investigating the role of the ETC in aging and age-related diseases: The ETC is implicated in the production of reactive oxygen species (ROS), which contribute to oxidative stress and aging. Research aims to understand this relationship and develop strategies for mitigating its effects.
The electron transport chain, with its complex interplay of proteins and electron carriers, and its reliance on oxygen as the crucial final electron acceptor, represents a fundamental process underpinning life itself. Further research will undoubtedly continue to unveil new facets of its function and potential for therapeutic intervention.
Latest Posts
Latest Posts
-
How Many Inches In Cubic Foot
Mar 31, 2025
-
A Person Who Study History Is Called
Mar 31, 2025
-
Is Calcium Oxide Ionic Or Covalent
Mar 31, 2025
-
What Is Not True Regarding Antibiotics
Mar 31, 2025
-
Balanced Equation For Copper And Nitric Acid
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Final Electron Acceptor In The Etc . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
