What Is The Expected Major Product For The Following Reaction
News Leon
Mar 16, 2025 · 5 min read
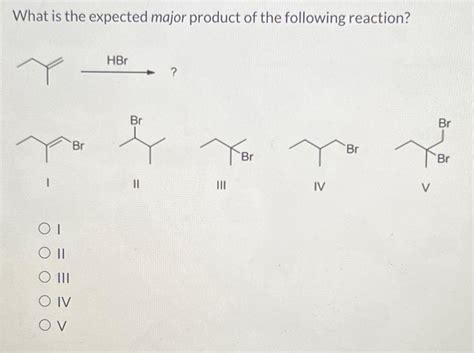
Table of Contents
Predicting the Major Product: A Comprehensive Guide to Organic Reaction Mechanisms
Predicting the major product of an organic reaction is a cornerstone of organic chemistry. It requires a deep understanding of reaction mechanisms, reaction kinetics, and the influence of various factors like sterics, electronics, and reaction conditions. This article delves into the process of predicting major products, using various examples to illustrate the key concepts. We'll cover several reaction types, exploring the nuances that determine the outcome.
Understanding Reaction Mechanisms: The Foundation of Prediction
Before we can predict the major product, we need to understand the mechanism of the reaction. The mechanism outlines the step-by-step process of bond breaking and bond formation, detailing the movement of electrons. This understanding allows us to identify the intermediates and transition states involved, crucial for predicting the most favorable pathway.
Key Factors Influencing Product Formation
Several factors play a crucial role in determining the major product:
-
Steric Hindrance: Bulky groups can hinder the approach of reagents, influencing reaction rates and directing the formation of less sterically hindered products.
-
Electronic Effects: Electron-donating and electron-withdrawing groups can significantly alter the reactivity of molecules, influencing the site of attack by electrophiles or nucleophiles. Resonance effects also play a significant role.
-
Reaction Conditions: Temperature, solvent, and the presence of catalysts or acids/bases can drastically affect the reaction pathway and product distribution.
-
Thermodynamics vs. Kinetics: The most stable product (thermodynamic product) is not always the major product. Sometimes, the kinetic product (formed faster) predominates, especially at lower temperatures.
Example Reactions and Predicting Major Products
Let's explore several common reaction types and discuss how to predict their major products:
1. Electrophilic Aromatic Substitution (EAS)
EAS reactions involve the substitution of a hydrogen atom on an aromatic ring with an electrophile. The position of substitution is heavily influenced by the substituents already present on the ring.
-
Activating, ortho/para-directing groups: These groups (e.g., -OH, -NH2, -OCH3) donate electron density to the ring, making it more reactive towards electrophiles and directing substitution to the ortho and para positions.
-
Deactivating, meta-directing groups: These groups (e.g., -NO2, -COOH, -SO3H) withdraw electron density from the ring, making it less reactive and directing substitution to the meta position.
Example: Nitration of toluene (methylbenzene). The methyl group is activating and ortho/para-directing. Therefore, the major products will be ortho-nitrotoluene and para-nitrotoluene, with the para isomer often predominating due to less steric hindrance.
2. Nucleophilic Substitution (SN1 and SN2)
Nucleophilic substitution reactions involve the replacement of a leaving group by a nucleophile. Two main mechanisms exist:
-
SN2: A concerted mechanism where the nucleophile attacks from the backside of the carbon atom bearing the leaving group, leading to inversion of configuration. Favored by strong nucleophiles, primary alkyl halides, and aprotic solvents.
-
SN1: A two-step mechanism involving the formation of a carbocation intermediate. Favored by weak nucleophiles, tertiary alkyl halides, and protic solvents. Often leads to racemization due to planar carbocation intermediate.
Example: Reaction of 2-bromobutane with sodium methoxide (NaOCH3) in methanol. Since 2-bromobutane is secondary, both SN1 and SN2 mechanisms are possible. However, the presence of a strong nucleophile (methoxide) and a protic solvent suggests that SN2 will be the dominant pathway, leading to inversion of configuration at the chiral center.
3. Elimination Reactions (E1 and E2)
Elimination reactions involve the removal of a leaving group and a proton from adjacent carbon atoms, leading to the formation of a double bond (alkene).
-
E2: A concerted mechanism requiring a strong base and often leading to Zaitsev's product (the most substituted alkene).
-
E1: A two-step mechanism involving the formation of a carbocation intermediate. Favored by weak bases and tertiary alkyl halides. Often leads to a mixture of products, including the Zaitsev product.
Example: Dehydration of 2-methyl-2-butanol with sulfuric acid. This reaction proceeds via an E1 mechanism, leading to the formation of 2-methyl-2-butene (Zaitsev product) as the major product due to its greater stability.
4. Addition Reactions
Addition reactions involve the addition of atoms or groups to a multiple bond (double or triple bond). The regioselectivity and stereoselectivity of addition reactions are crucial for predicting the major product.
-
Markovnikov's rule: In the addition of HX to an alkene, the hydrogen atom adds to the carbon atom with more hydrogen atoms already attached.
-
Anti-Markovnikov's addition: In the presence of peroxides, the addition of HX to an alkene proceeds via a radical mechanism, resulting in the opposite regioselectivity (anti-Markovnikov addition).
Example: Addition of HBr to propene. According to Markovnikov's rule, the major product will be 2-bromopropane.
5. Aldol Condensation
Aldol condensation involves the reaction of an aldehyde or ketone with another aldehyde or ketone in the presence of a base, leading to the formation of a β-hydroxyaldehyde or β-hydroxyketone. Dehydration can often follow, leading to an α,β-unsaturated carbonyl compound.
Example: Aldol condensation of acetaldehyde. The reaction will produce 3-hydroxybutanal, which can then undergo dehydration to form crotonaldehyde (but-2-enal).
Advanced Considerations: A Deeper Dive
Predicting the major product often requires a more nuanced understanding that considers:
-
Kinetic vs. Thermodynamic Control: As mentioned earlier, the fastest reaction pathway doesn't always produce the most stable product. Reaction conditions strongly influence this aspect.
-
Transition State Theory: Understanding the energy profile of the reaction pathway, including the activation energies of various steps, can provide insights into the relative rates of different pathways.
-
Computational Chemistry: Advanced computational methods can be used to model reactions and predict their outcomes, providing detailed information about reaction pathways and energy barriers.
Conclusion: Mastering the Art of Prediction
Predicting the major product of an organic reaction is a complex but rewarding skill. It involves understanding reaction mechanisms, considering various influencing factors, and applying knowledge of organic chemistry principles. By systematically analyzing the reaction conditions and the properties of the reactants, we can accurately predict the most likely outcome. This ability is critical for designing and executing synthetic strategies in organic chemistry research and development. Continuous practice and a deep understanding of the underlying principles are essential for mastering this vital skill. The examples provided here serve as a starting point for your journey toward becoming proficient in predicting major products in organic reactions. Remember, each reaction is unique, and careful consideration of all relevant factors is crucial for accurate predictions.
Latest Posts
Latest Posts
-
Is Cl An Acid Or Base
Mar 17, 2025
-
A Charge Of Uniform Linear Density
Mar 17, 2025
-
Which Of The Following Is Polynomial
Mar 17, 2025
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Expected Major Product For The Following Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
