What Is The Empirical Formula Of Magnesium Oxide
News Leon
Mar 16, 2025 · 6 min read
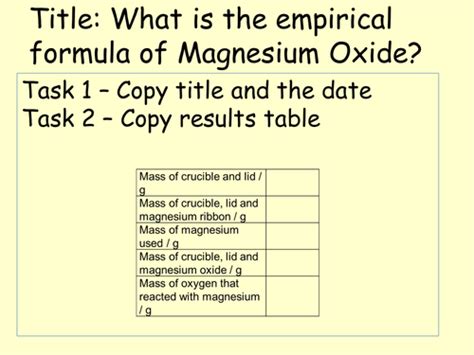
Table of Contents
What is the Empirical Formula of Magnesium Oxide? A Comprehensive Guide
Determining the empirical formula of magnesium oxide (MgO) is a classic chemistry experiment that beautifully illustrates the principles of stoichiometry and experimental techniques. This guide dives deep into the process, exploring the theoretical background, practical steps involved, and potential sources of error, ensuring a thorough understanding of this fundamental concept.
Understanding Empirical Formulas and Magnesium Oxide
Before delving into the experimental process, let's clarify some crucial concepts. An empirical formula represents the simplest whole-number ratio of atoms of each element present in a compound. It doesn't necessarily reflect the actual arrangement or number of atoms in a molecule (which is given by the molecular formula).
Magnesium oxide (MgO) is an ionic compound formed by the reaction between magnesium (Mg), an alkaline earth metal, and oxygen (O), a non-metal. Magnesium readily loses two electrons to achieve a stable electron configuration, forming a Mg²⁺ cation. Oxygen readily gains two electrons to achieve a stable configuration, forming an O²⁻ anion. The electrostatic attraction between these oppositely charged ions leads to the formation of the ionic compound MgO. The strong ionic bonds contribute to MgO's high melting point and crystalline structure.
Determining the Empirical Formula Through Experimentation
The empirical formula of magnesium oxide can be determined experimentally by carefully measuring the mass of magnesium used and the mass of magnesium oxide produced after a controlled reaction with oxygen. This allows us to calculate the ratio of moles of magnesium to moles of oxygen in the compound. Here's a breakdown of the experimental procedure:
1. Materials Required:
- Magnesium ribbon: A precise mass is crucial for accurate results.
- Bunsen burner: Provides the heat necessary for the reaction.
- Crucible and lid: A crucible is a heat-resistant container for the reaction. The lid helps to control the oxygen flow.
- Clay triangle: Supports the crucible during heating.
- Tripod stand: Provides a stable base for the Bunsen burner and clay triangle.
- Spatula: For handling the magnesium ribbon.
- Electronic balance: For precise mass measurements.
- Tongs: For safely handling the hot crucible.
- Goggles and safety gloves: Essential safety precautions.
2. Experimental Procedure:
- Clean and weigh the crucible and lid: This initial measurement is crucial for subtracting the mass of the crucible later. Record the combined mass with high precision.
- Add magnesium ribbon: Carefully add a measured mass (around 0.2-0.5 grams) of magnesium ribbon to the crucible. Record the mass of the crucible, lid, and magnesium precisely.
- Heat the crucible gently: Initially heat the crucible gently to prevent the magnesium from reacting too rapidly. This step helps to ensure complete combustion of the magnesium. This should be done using a low blue flame and constant monitoring.
- Increase heat gradually: As the magnesium begins to react (you’ll see a bright white light), increase the heat gradually to ensure complete conversion of magnesium to magnesium oxide. The reaction is exothermic, and heat needs careful control.
- Continue heating until constant mass: Continue heating the crucible and its contents until there is no further change in mass (i.e., the mass remains constant after repeated heating and cooling cycles). This indicates that the magnesium has completely reacted with oxygen to form MgO. Weigh the crucible, lid, and magnesium oxide carefully. Allow it to cool completely before weighing to avoid errors due to heat expansion.
- Calculations: Subtract the initial mass of the crucible and lid from the final mass (crucible + lid + MgO) to determine the mass of magnesium oxide produced.
3. Data Analysis and Calculations:
After completing the experiment, perform the following calculations:
- Mass of oxygen reacted: Subtract the mass of magnesium from the mass of magnesium oxide to find the mass of oxygen that reacted.
- Moles of magnesium: Divide the mass of magnesium by its molar mass (24.31 g/mol).
- Moles of oxygen: Divide the mass of oxygen by its molar mass (16.00 g/mol).
- Mole ratio: Divide the number of moles of magnesium by the number of moles of oxygen. This ratio should be approximately 1:1, confirming the empirical formula MgO.
- Empirical Formula: Based on the mole ratio (ideally 1:1), write the empirical formula. If it is not precisely 1:1 (experimental errors are common), express the ratio in its simplest whole-number form.
Potential Sources of Error and Mitigation Strategies
Several factors can introduce errors into the experimental determination of the empirical formula of magnesium oxide. Understanding these potential sources of error and implementing appropriate mitigation strategies is crucial for obtaining accurate and reliable results.
- Incomplete Reaction: If the magnesium doesn't completely react with oxygen, the calculated mass of magnesium oxide will be too low, leading to an incorrect empirical formula. This can be mitigated by ensuring sufficient heating and allowing sufficient reaction time.
- Formation of Magnesium Nitride: Magnesium can react with nitrogen in the air to form magnesium nitride (Mg₃N₂). This side reaction can alter the final mass and skew the results. Using a crucible lid helps to minimize the reaction with nitrogen. Heating in a controlled oxygen environment would further minimize this issue.
- Absorption of Moisture: Magnesium oxide is hygroscopic, meaning it absorbs moisture from the air. Weighing the magnesium oxide while it’s still hot prevents accurate mass determination. Allow it to cool in a desiccator to minimize moisture absorption before weighing.
- Weighing Errors: Inaccurate weighing of the magnesium ribbon and the final product can significantly impact the results. Using a high-precision electronic balance and carefully calibrating it will help reduce weighing errors. Repeated weighings and averaging results can further mitigate these errors.
- Improper Heating: Insufficient heating can lead to an incomplete reaction. Overheating can cause the magnesium oxide to decompose. Controlling the heating rate is crucial for accuracy. A Bunsen burner with proper adjustment for flame size and heat control is recommended.
Conclusion: Understanding the Empirical Formula of Magnesium Oxide
The empirical formula of magnesium oxide, MgO, is a fundamental concept in chemistry, illustrating the relationship between mass, moles, and the composition of a compound. This experiment allows students to gain hands-on experience with stoichiometric calculations, data analysis, and error analysis. By understanding the experimental procedure, potential sources of error, and the mitigation strategies, students can conduct the experiment effectively and accurately determine the empirical formula, thereby improving their experimental skills and deepening their understanding of stoichiometry. The experiment emphasizes the importance of careful experimental techniques and meticulous data analysis for achieving accurate results. Further investigation might include comparing results from multiple trials to evaluate experimental error and precision. Understanding the limitations and inherent uncertainties in experimental data is crucial for interpreting scientific results accurately.
Latest Posts
Latest Posts
-
What Is A Hot Shot Drugs
Mar 16, 2025
-
What Is A Property Of An Ionic Compound
Mar 16, 2025
-
What Is Liquid To Solid Called
Mar 16, 2025
-
How Many Atp Are Produced In Anaerobic Respiration
Mar 16, 2025
-
Identify The Correct And Incorrect Statements
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about What Is The Empirical Formula Of Magnesium Oxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
