What Is Liquid To Solid Called
News Leon
Mar 16, 2025 · 6 min read
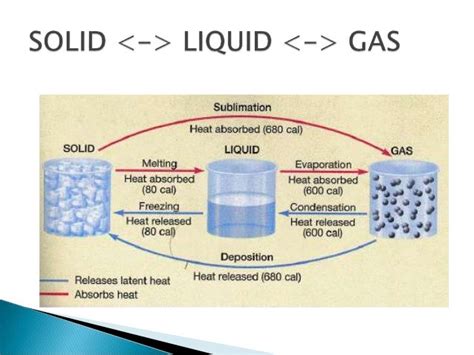
Table of Contents
What is Liquid to Solid Called? Exploring the Fascinating World of Solidification
The transformation of a liquid into a solid is a fundamental process in nature and industry, crucial to everything from the formation of rocks to the creation of everyday materials. Understanding this phase transition, known as solidification, requires exploring various aspects, from the underlying physics and chemistry to the diverse applications and techniques involved. This comprehensive guide delves into the intricacies of liquid-to-solid transitions, examining the different types, influencing factors, and practical implications.
Understanding the Basics: Freezing, Solidification, and Crystallization
While often used interchangeably, the terms "freezing," "solidification," and "crystallization" carry nuanced meanings within the context of phase transitions.
Freezing typically refers to the solidification of a liquid at its freezing point, a specific temperature at which the liquid and solid phases coexist in equilibrium. This transition is often associated with water turning into ice, but it applies to a broad range of substances. Freezing point depression, where the freezing point is lowered by adding solutes, is a key concept in various applications.
Solidification is a broader term encompassing all processes where a liquid transforms into a solid, regardless of the mechanism or temperature. It includes both freezing and other forms of solid formation, such as the setting of cement or the hardening of polymers.
Crystallization, a specific type of solidification, involves the formation of a crystalline solid structure. Crystals are characterized by their ordered, repeating atomic arrangements, resulting in distinct geometrical shapes and properties. Many materials, like metals and salts, solidify through crystallization, leading to materials with unique characteristics. Amorphous solids, on the other hand, lack this ordered structure, exhibiting properties different from their crystalline counterparts.
The Physics and Chemistry Behind Solidification
The transition from a liquid to a solid hinges on the interplay of intermolecular forces and kinetic energy.
-
Intermolecular Forces: In liquids, molecules possess sufficient kinetic energy to overcome the attractive forces between them, allowing for relatively free movement. As the temperature drops, kinetic energy decreases, and intermolecular forces become dominant. These forces, such as van der Waals forces, hydrogen bonds, and ionic interactions, pull molecules together, leading to a more ordered structure.
-
Nucleation and Crystal Growth: Solidification typically begins with nucleation, the formation of tiny solid particles within the liquid. These nuclei act as seeds for further crystal growth. The rate of nucleation significantly influences the final microstructure of the solid. Homogeneous nucleation occurs spontaneously within the liquid, while heterogeneous nucleation involves nucleation on existing surfaces, like impurities or container walls.
-
Crystal Growth: Once nuclei are formed, they grow by the addition of molecules from the surrounding liquid. The rate of crystal growth depends on factors like temperature, cooling rate, and the presence of impurities. Rapid cooling often leads to smaller, less perfectly formed crystals, while slow cooling allows for larger, more well-defined crystals.
-
Latent Heat of Fusion: During solidification, the liquid releases energy known as the latent heat of fusion. This heat must be removed for the phase transition to complete. The rate of heat removal significantly influences the solidification process. Efficient heat removal promotes faster solidification and potentially better crystal structure.
Types of Solidification Processes
The methods and techniques used for solidifying liquids vary depending on the material and desired properties. Here are some notable examples:
-
Casting: A widely used technique, casting involves pouring molten material into a mold, allowing it to solidify into the desired shape. Various casting methods exist, including sand casting, die casting, investment casting, and continuous casting, each offering unique advantages and applications.
-
Freezing: The simplest form of solidification, freezing relies on lowering the temperature below the freezing point. This method is prevalent in food preservation, cryogenics, and the production of ice.
-
Spray Drying: Used extensively in the food and pharmaceutical industries, spray drying involves atomizing a liquid into fine droplets, which then solidify as they come into contact with a stream of hot air.
-
Polymerization: Polymerization is a chemical process where smaller molecules (monomers) combine to form larger chain-like molecules (polymers). Many polymers solidify through polymerization, resulting in a wide range of materials with diverse properties.
-
3D Printing (Additive Manufacturing): This advanced technique builds solid objects layer by layer from a liquid or paste-like material, offering unprecedented design flexibility and precision.
Factors Affecting Solidification
Several factors influence the solidification process, impacting the properties of the resulting solid:
-
Temperature: The rate of cooling significantly affects the final structure and properties of the solid. Rapid cooling often leads to smaller grains and a higher degree of disorder, while slow cooling allows for larger, more well-ordered grains.
-
Pressure: Pressure can affect the freezing point of a material. Increased pressure generally raises the freezing point of most substances, but exceptions exist, like water.
-
Impurities: The presence of impurities in the liquid can significantly influence nucleation and crystal growth, leading to changes in the solid's microstructure and properties.
-
Cooling Rate: The speed at which the liquid is cooled directly impacts crystal size and perfection. Slow cooling favors larger, more ordered crystals, while rapid cooling results in smaller, less perfect crystals.
-
Convection: Movement within the liquid during solidification can affect heat transfer and the uniformity of the resulting solid.
Applications of Solidification
Solidification processes are fundamental to a vast array of industries and applications:
-
Materials Science and Engineering: The solidification of metals, alloys, and ceramics is crucial for producing a wide range of structural materials, from aircraft components to building materials. Understanding solidification processes is essential for controlling the microstructure and achieving desired mechanical properties.
-
Food Processing: Freezing is a common method of food preservation, extending shelf life and maintaining food quality. Understanding freezing kinetics is crucial for minimizing ice crystal formation, which can damage food texture.
-
Pharmaceutical Industry: Solidification plays a key role in drug formulation and delivery. The formation of tablets, capsules, and other dosage forms relies on controlled solidification processes.
-
Cryogenics: Cryogenics utilizes extremely low temperatures to study material properties and develop advanced technologies. Solidification at cryogenic temperatures is vital in these applications.
-
Geology and Planetary Science: The solidification of magma and lava plays a crucial role in the formation of igneous rocks, shaping the Earth's crust and other planetary bodies.
-
Manufacturing: Numerous manufacturing processes rely on solidification, including casting, molding, and 3D printing.
Advanced Techniques and Research
Ongoing research continues to refine and expand our understanding of solidification processes. Advanced techniques such as:
-
Computational Modeling: Computational methods, such as molecular dynamics simulations, provide insights into the atomic-level mechanisms of solidification, allowing for the prediction of microstructure and properties.
-
In-situ Observation: Advanced techniques for observing solidification in real-time provide valuable data on the nucleation, growth, and microstructure evolution.
-
Microgravity Experiments: Experiments conducted in microgravity environments minimize the effects of convection, enabling a better understanding of fundamental solidification phenomena.
Conclusion: The Ongoing Significance of Solidification
The transformation from liquid to solid is a fundamental physical process with far-reaching implications across diverse fields. From the formation of geological structures to the manufacturing of advanced materials, understanding the intricacies of solidification is paramount. Continued research and technological advancements continue to refine our knowledge, opening new possibilities in materials science, engineering, and numerous other disciplines. The seemingly simple process of solidification remains a complex and fascinating area of study, vital to our understanding of the world around us and the development of future technologies.
Latest Posts
Latest Posts
-
An Earth Satellite Moves In A Circular Orbit
Mar 17, 2025
-
What Is The Value Of K In Physics
Mar 17, 2025
-
The Study Of Tissues With A Microscope Is Called
Mar 17, 2025
-
The Male Gamete Is Called The
Mar 17, 2025
-
The Broad Portion Of The Leaf That Carries Out Photosynthesis
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is Liquid To Solid Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
