What Is A Property Of An Ionic Compound
News Leon
Mar 16, 2025 · 7 min read
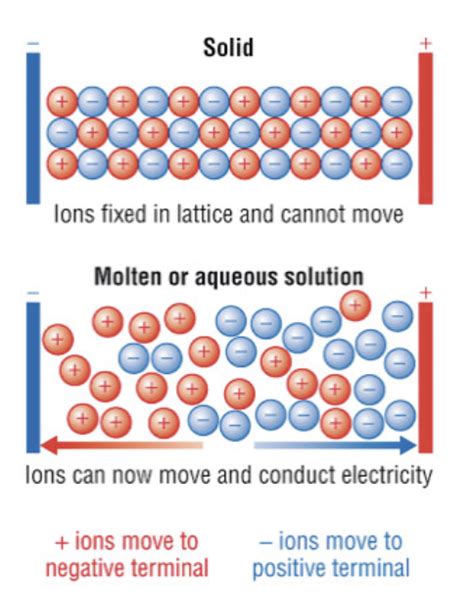
Table of Contents
What is a Property of an Ionic Compound? A Deep Dive into the Characteristics of Ionic Substances
Ionic compounds, formed through the electrostatic attraction between oppositely charged ions, exhibit a unique set of properties that distinguish them from other types of chemical compounds, such as covalent compounds. Understanding these properties is crucial in various fields, from materials science to medicine. This comprehensive guide delves deep into the characteristics of ionic compounds, exploring their formation, structure, and behavior.
Defining Ionic Compounds: A Foundation for Understanding
Before exploring the specific properties, let's establish a clear definition. An ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonds. These bonds arise from the transfer of electrons from one atom (typically a metal) to another (typically a non-metal), resulting in the formation of positively charged cations and negatively charged anions. The strong electrostatic attraction between these oppositely charged ions is what gives ionic compounds their distinctive properties. The simplest example is sodium chloride (NaCl), or common table salt, where sodium (Na) loses an electron to become a positively charged Na⁺ ion and chlorine (Cl) gains an electron to become a negatively charged Cl⁻ ion. This transfer of electrons creates a strong ionic bond between the two ions.
Key Players in Ionic Bond Formation: Metals and Non-metals
The formation of ionic compounds is primarily driven by the electronegativity difference between the participating atoms. Electronegativity refers to an atom's ability to attract electrons towards itself in a chemical bond. Metals, generally having low electronegativity, tend to lose electrons readily, becoming positively charged cations. Non-metals, possessing high electronegativity, tend to gain electrons, becoming negatively charged anions. The larger the difference in electronegativity between a metal and a non-metal, the stronger the ionic bond will be.
Exploring the Key Properties of Ionic Compounds
Now, let's explore the defining properties that set ionic compounds apart:
1. High Melting and Boiling Points: The Strength of Ionic Bonds
One of the most prominent properties of ionic compounds is their exceptionally high melting and boiling points. This is a direct consequence of the strong electrostatic forces holding the ions together in a crystal lattice. To melt or boil an ionic compound, a significant amount of energy is required to overcome these strong attractive forces and break the ionic bonds. This explains why many ionic compounds exist as solids at room temperature. The stronger the ionic bond (which correlates with higher charge and smaller ion size), the higher the melting and boiling points will be.
2. Crystalline Structure: An Ordered Arrangement of Ions
Ionic compounds typically exist as crystalline solids with a highly ordered arrangement of ions in a three-dimensional lattice structure. This regular arrangement maximizes the electrostatic attractions between the oppositely charged ions, minimizing the repulsive forces between like charges. The specific arrangement of ions depends on the relative sizes and charges of the cations and anions, leading to different crystal structures (e.g., cubic close-packed, body-centered cubic). This ordered structure contributes significantly to their hardness and brittleness.
3. Hardness and Brittleness: A Consequence of Crystal Structure
The strong electrostatic forces in the crystal lattice also contribute to the hardness of ionic compounds. However, this ordered structure also makes them brittle. When subjected to stress, the layers of ions in the crystal lattice can shift, bringing ions of like charges into close proximity. This results in strong repulsive forces that cause the crystal to fracture along cleavage planes. This explains why ionic crystals tend to shatter rather than deform under pressure.
4. Solubility in Polar Solvents: The Role of Dipoles
Ionic compounds are often soluble in polar solvents like water, but generally insoluble in non-polar solvents like oil. This solubility arises from the interaction between the polar solvent molecules and the ions in the crystal lattice. Water molecules, for instance, possess a dipole moment—a separation of positive and negative charges—that allows them to interact strongly with the ions. The partially positive end of the water molecule attracts the anions, while the partially negative end attracts the cations, effectively surrounding the ions and pulling them away from the crystal lattice. This process, called hydration, leads to the dissolution of the ionic compound.
5. Electrical Conductivity: Conduction in Solution and Molten State
Ionic compounds are generally poor conductors of electricity in their solid state because the ions are fixed in the crystal lattice and cannot move freely to carry the charge. However, they become excellent conductors when dissolved in water or melted. In solution or molten state, the ions are free to move, allowing them to carry an electric current. The movement of these charged particles under an electric field constitutes the electrical conductivity.
6. High Enthalpy of Solution: Energy Changes During Dissolution
The enthalpy of solution, which represents the heat change when a substance dissolves, is often substantial for ionic compounds. This is because energy is required to overcome the strong ionic bonds in the crystal lattice and to hydrate the ions. The overall enthalpy change can be either exothermic (releasing heat) or endothermic (absorbing heat), depending on the relative magnitudes of the lattice energy (energy required to break the ionic bonds) and the hydration energy (energy released when ions are hydrated).
Factors Influencing Ionic Compound Properties: A Closer Look
Several factors influence the specific properties of an ionic compound:
1. Charge of Ions: The Magnitude of Electrostatic Attraction
The magnitude of the charges on the ions significantly impacts the strength of the ionic bond. Higher charges lead to stronger electrostatic attractions, resulting in higher melting and boiling points, increased hardness, and reduced solubility. For example, magnesium oxide (MgO), with Mg²⁺ and O²⁻ ions, has a higher melting point than sodium chloride (NaCl), with Na⁺ and Cl⁻ ions.
2. Size of Ions: The Distance Between Charges
The size of the ions also plays a crucial role. Smaller ions lead to stronger electrostatic attractions because the charges are closer together. Conversely, larger ions result in weaker ionic bonds and lower melting and boiling points.
3. Lattice Energy: The Energy Required to Break the Lattice
Lattice energy is the energy required to separate one mole of an ionic compound into its gaseous ions. It is a measure of the strength of the ionic bonds in the crystal lattice. Higher lattice energy indicates stronger ionic bonds, resulting in higher melting points and lower solubility.
Applications of Ionic Compounds: A Wide Range of Uses
The unique properties of ionic compounds make them indispensable in various applications:
-
Medicine: Many ionic compounds are essential for biological processes and are used in pharmaceuticals. For example, sodium chloride is crucial for maintaining electrolyte balance in the body. Other ionic compounds are used as medications or in drug delivery systems.
-
Industry: Ionic compounds are widely used in various industrial processes. Sodium hydroxide (NaOH), for instance, is a crucial component in many chemical manufacturing processes. Other examples include the use of ionic compounds in fertilizers, pigments, and detergents.
-
Materials Science: The properties of ionic compounds are exploited in materials science to create materials with specific characteristics. For example, certain ionic compounds are used in high-temperature applications due to their high melting points, while others are used in optical devices due to their unique optical properties.
Conclusion: A Summary of Ionic Compound Properties
In conclusion, ionic compounds possess a distinctive set of properties arising from the strong electrostatic attractions between their constituent ions. These properties, including high melting and boiling points, crystalline structure, hardness and brittleness, solubility in polar solvents, electrical conductivity in solution and molten state, and high enthalpy of solution, make them valuable in a wide array of applications. Understanding these properties is crucial for predicting the behavior of ionic compounds and for developing new materials and technologies based on them. Further exploration into the intricacies of ionic bonding and crystal structure reveals an even deeper appreciation for the fascinating world of ionic compounds and their diverse applications.
Latest Posts
Latest Posts
-
Which Bond Is The Most Polar
Mar 17, 2025
-
Burning Of Candle Is Chemical Change
Mar 17, 2025
-
An Earth Satellite Moves In A Circular Orbit
Mar 17, 2025
-
What Is The Value Of K In Physics
Mar 17, 2025
-
The Study Of Tissues With A Microscope Is Called
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is A Property Of An Ionic Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
