What Is The Empirical Formula Of C6h12o6
News Leon
Mar 25, 2025 · 5 min read
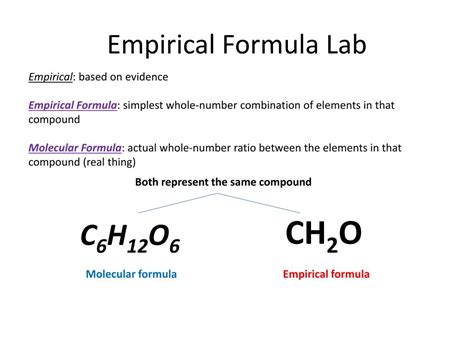
Table of Contents
What is the Empirical Formula of C₆H₁₂O₆? Understanding Molecular and Empirical Formulas
The question, "What is the empirical formula of C₆H₁₂O₆?" might seem deceptively simple at first glance. However, understanding the answer requires a grasp of fundamental chemistry concepts, specifically the difference between molecular and empirical formulas. This article will delve deep into this topic, explaining these concepts, calculating empirical formulas, and examining the specific case of C₆H₁₂O₆, a crucial molecule in biology.
Molecular vs. Empirical Formulas: A Crucial Distinction
Before tackling the empirical formula of C₆H₁₂O₆, it’s essential to understand the difference between molecular and empirical formulas. Both represent the composition of a compound, but they do so in different ways:
-
Molecular Formula: This formula indicates the exact number of each type of atom present in a molecule. It’s the true representation of the molecule's composition. For example, the molecular formula of glucose is C₆H₁₂O₆, meaning each glucose molecule contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
-
Empirical Formula: This formula represents the simplest whole-number ratio of atoms in a compound. It shows the relative proportions of different atoms, but not necessarily the exact number in a single molecule. It's the smallest possible whole-number ratio of atoms in a compound.
Calculating Empirical Formulas: A Step-by-Step Guide
Calculating an empirical formula usually involves experimental data, like the mass percentage composition of elements in a compound. Let's outline the general steps:
-
Determine the mass percentage of each element: This information is often provided in the problem statement or obtained through laboratory analysis (e.g., combustion analysis).
-
Assume a 100g sample: This simplifies calculations; the percentages directly become grams.
-
Convert grams to moles: Using the molar mass of each element (found on the periodic table), convert the grams of each element to moles.
-
Find the mole ratio: Divide the number of moles of each element by the smallest number of moles calculated. This gives the simplest whole-number ratio of atoms.
-
Express as a formula: Write the empirical formula using the whole-number ratios as subscripts for each element.
The Empirical Formula of C₆H₁₂O₆: A Case Study
Now, let's apply this knowledge to the molecule C₆H₁₂O₆, commonly known as glucose. The molecular formula clearly shows six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. To find the empirical formula, we need to determine the simplest whole-number ratio.
We can do this by dividing all subscripts by their greatest common divisor, which in this case is 6:
- Carbon: 6 / 6 = 1
- Hydrogen: 12 / 6 = 2
- Oxygen: 6 / 6 = 1
Therefore, the empirical formula of C₆H₁₂O₆ is CH₂O. This means that the simplest whole-number ratio of atoms in glucose is one carbon atom, two hydrogen atoms, and one oxygen atom. It is important to note that many other sugars also share this same empirical formula.
The Importance of Glucose (C₆H₁₂O₆) in Biology
Glucose (C₆H₁₂O₆) is a fundamental molecule in biology, playing several crucial roles:
-
Energy Source: Glucose is the primary source of energy for most living organisms. It is broken down through cellular respiration, releasing energy in the form of ATP (adenosine triphosphate), which fuels cellular processes.
-
Building Block: Glucose serves as a building block for larger molecules such as starch, cellulose, and glycogen. Plants store glucose as starch, animals as glycogen, and plants use cellulose as a structural component in cell walls.
-
Metabolic Intermediate: Glucose participates in numerous metabolic pathways, acting as an intermediate in the synthesis and breakdown of various biomolecules.
-
Blood Sugar: The level of glucose in the blood is tightly regulated, and its concentration plays a critical role in maintaining overall health. Diabetes, for example, is characterized by abnormal blood glucose levels.
Other Molecules with the Empirical Formula CH₂O
It's crucial to emphasize that the empirical formula CH₂O is not unique to glucose. Several other molecules share this same empirical formula, including:
-
Formaldehyde (HCHO): A simple aldehyde, formaldehyde is a pungent gas used in various industrial applications. Its molecular formula is the same as its empirical formula.
-
Acetic Acid (CH₃COOH): A common organic acid, acetic acid is the main component of vinegar. Its molecular formula is C₂H₄O₂, which simplifies to the empirical formula CH₂O.
-
Various Sugars: Many other monosaccharides and polysaccharides have the empirical formula CH₂O.
Distinguishing Molecules with the Same Empirical Formula
The fact that different molecules can share the same empirical formula highlights the importance of the molecular formula. The empirical formula alone doesn't provide enough information to identify a specific compound. To distinguish between molecules with identical empirical formulas, additional information is necessary, such as:
-
Molecular weight: This allows the determination of the actual molecular formula.
-
Structural analysis: Techniques such as NMR (Nuclear Magnetic Resonance) and IR (Infrared) spectroscopy help determine the arrangement of atoms within the molecule.
-
Chemical properties: Different molecules exhibit different chemical properties, even if they have the same empirical formula.
Conclusion: Empirical Formula as a Tool, Not a Complete Identity
The empirical formula of C₆H₁₂O₆ is CH₂O, reflecting the simplest whole-number ratio of atoms in the molecule. However, this is only a simplified representation. The molecular formula, C₆H₁₂O₆, is essential for accurately identifying glucose and distinguishing it from other molecules with the same empirical formula. Understanding both molecular and empirical formulas is vital for comprehending chemical composition and the behavior of substances. This distinction emphasizes the multifaceted nature of chemical analysis and the importance of combining different analytical techniques for a complete and accurate understanding of molecular structure and identity.
Latest Posts
Latest Posts
-
When Lithium Hydroxide Pellets Are Added
Mar 26, 2025
-
Common Factors Of 36 And 54
Mar 26, 2025
-
Inventory Is Classified On The Balance Sheet As A
Mar 26, 2025
-
What Is The Opposite Of Hyperbole
Mar 26, 2025
-
Major And Minor Grooves In Dna
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Empirical Formula Of C6h12o6 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
