What Is The Electron Configuration For Co
News Leon
Mar 18, 2025 · 5 min read
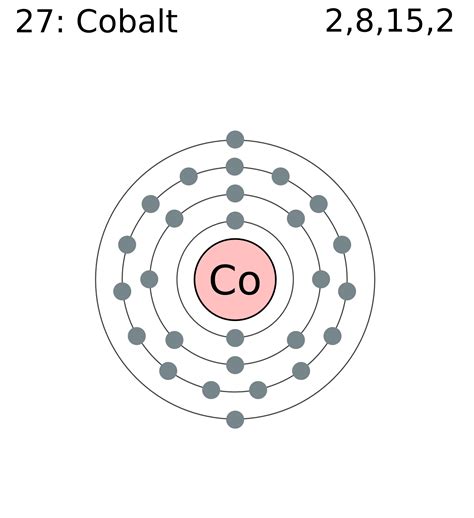
Table of Contents
What is the Electron Configuration for Cobalt (Co)? A Deep Dive into Atomic Structure
Understanding the electron configuration of an element is fundamental to comprehending its chemical behavior and properties. Cobalt (Co), a transition metal with a rich history and diverse applications, presents a fascinating case study in electron configuration. This article will delve deep into the electron configuration of cobalt, exploring the underlying principles, exceptions, and implications of its unique electronic structure.
Understanding Electron Configuration Basics
Before we dive into the specifics of cobalt, let's establish a foundational understanding of electron configuration. Electron configuration describes the arrangement of electrons within the various energy levels and sublevels of an atom. It follows specific rules dictated by quantum mechanics:
- Aufbau Principle: Electrons fill orbitals starting with the lowest energy levels and progressing upwards. This is often visualized using the Aufbau diagram or filling order diagram.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, with opposite spins. This is often represented by up and down arrows in orbital diagrams.
- Hund's Rule: Within a subshell (e.g., p, d, f), electrons will individually occupy each orbital before pairing up in any one orbital. This maximizes the total spin and stability of the atom.
These three principles guide us in predicting the electron configuration of any element.
Determining the Electron Configuration of Cobalt (Co)
Cobalt has an atomic number of 27, meaning it has 27 protons and 27 electrons in its neutral state. To determine its electron configuration, we follow the Aufbau principle, filling orbitals in increasing order of energy:
1s², 2s², 2p⁶, 3s², 3p⁶, 4s², 3d⁷
This configuration can also be written in a condensed form using the noble gas configuration of Argon (Ar):
[Ar] 4s² 3d⁷
Let's break down this configuration:
-
[Ar]: This represents the electron configuration of Argon (1s²2s²2p⁶3s²3p⁶), which is a stable noble gas configuration. This notation simplifies the representation by focusing on the electrons beyond the noble gas core.
-
4s²: Two electrons occupy the 4s orbital. The 4s orbital is generally filled before the 3d orbital, despite the 3d having a slightly lower principal quantum number, due to subtle differences in energy levels.
-
3d⁷: Seven electrons occupy the 3d orbitals. Following Hund's rule, these electrons individually occupy each of the five 3d orbitals before pairing up. This results in three orbitals with single electrons and two orbitals with paired electrons. This partially filled d-subshell is crucial in determining cobalt's magnetic and chemical properties.
Implications of Cobalt's Electron Configuration
Cobalt's [Ar] 4s² 3d⁷ electron configuration has significant implications for its physical and chemical properties:
Magnetic Properties:
The partially filled 3d subshell is responsible for cobalt's ferromagnetism. The unpaired electrons in the 3d orbitals interact with each other, aligning their spins and creating a strong magnetic field. This property makes cobalt a vital component in magnets and magnetic storage devices.
Chemical Properties:
The presence of unpaired electrons in the 3d orbitals makes cobalt a highly reactive transition metal. It readily forms a variety of compounds with varying oxidation states, most commonly +2 and +3. The 4s electrons are typically lost first in chemical reactions. The ability to have variable oxidation states contributes to the diverse chemistry of cobalt compounds.
Catalytic Activity:
Cobalt's unique electron configuration makes it an excellent catalyst in various chemical reactions. The ability of the d orbitals to accommodate electrons at different energy levels allows cobalt to participate in redox reactions, facilitating the transformation of reactants into products. Cobalt catalysts are employed in numerous industrial processes, including the production of plastics, synthetic fuels, and pharmaceuticals.
Exceptions to the Aufbau Principle and Cobalt
While the Aufbau principle provides a reliable framework for predicting electron configurations, there are exceptions, particularly among transition metals. Although cobalt generally follows the Aufbau principle, it’s important to note that subtle energy level variations can sometimes lead to minor deviations. The general trend is fairly consistent, however.
Cobalt's Role in Biology and Technology
Cobalt's unique properties, stemming directly from its electron configuration, make it essential in various biological and technological applications.
Vitamin B12:
Cobalt is a crucial component of vitamin B12 (cobalamin), a vital coenzyme involved in numerous metabolic processes, including DNA synthesis and red blood cell formation. The cobalt ion in vitamin B12 is coordinated to a corrin ring, a modified porphyrin structure, and its specific electronic configuration within this complex is critical for its biological function.
Alloys and Materials Science:
Cobalt's magnetic properties and high strength make it a valuable component in various alloys, including stainless steel and superalloys. These alloys are used in a wide range of applications, from kitchen utensils to jet engine components. The precise electron configuration contributes to the unique combination of strength, corrosion resistance, and magnetic properties displayed by these materials.
Pigments and Catalysts:
Cobalt compounds are also used as pigments in paints, ceramics, and glass. The color of these pigments is determined by the oxidation state of cobalt and its coordination environment. Cobalt's catalytic activity is also exploited in various industrial processes, such as Fischer-Tropsch synthesis and hydroformylation.
Conclusion: A Deeper Understanding of Cobalt
The electron configuration of cobalt ([Ar] 4s² 3d⁷) is not merely a theoretical construct; it is the fundamental basis for its remarkable properties and diverse applications. Understanding this configuration helps us appreciate cobalt's role in magnetism, catalysis, biology, and technology. The intricate interplay of the Aufbau principle, Hund's rule, and the subtle variations in energy levels contributes to the fascinating chemistry and significant importance of this transition metal. Further exploration of cobalt's chemistry and its implications for various fields is ongoing, continually uncovering new applications and deeper understanding of this versatile element. This deeper understanding strengthens the ability to leverage cobalt's unique properties for advancements in numerous technological sectors and provides a strong foundation for continuing research in various scientific fields.
Latest Posts
Latest Posts
-
What Is 25 Percent Of 25
Mar 19, 2025
-
How Do You Make A Magnet At Home
Mar 19, 2025
-
A Decrease In Demand And An Increase In Supply Will
Mar 19, 2025
-
Prove The Square Root Of 5 Is Irrational
Mar 19, 2025
-
The Figure Shown Is A Rectangle With A Semicircle
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Co . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
