What Is The Difference Between Ml And Mg
News Leon
Mar 16, 2025 · 5 min read
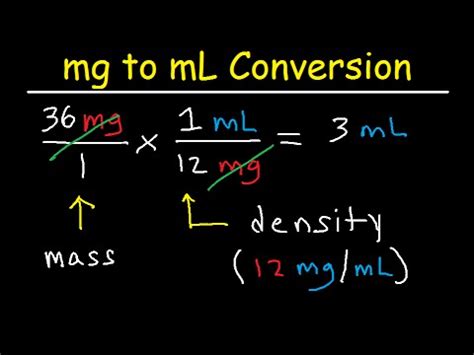
Table of Contents
What's the Difference Between mL and mg? A Comprehensive Guide
The seemingly simple question, "What's the difference between mL and mg?" often trips up even those with some scientific background. The confusion arises because both units are used to measure things, but they measure fundamentally different properties. Understanding the core difference is crucial, not just for scientific accuracy but also for safety, especially when dealing with medication or chemical solutions. This comprehensive guide will clarify the distinction between mL (milliliters) and mg (milligrams), exploring their definitions, applications, and the potential dangers of misinterpreting them.
Understanding Units of Measurement: Volume vs. Mass
The root of the confusion lies in understanding the core concepts of volume and mass.
Volume: How Much Space Something Occupies
mL (milliliter) is a unit of volume. Volume measures the amount of three-dimensional space occupied by a substance. Think of it as the size of a container or the space filled by a liquid. A milliliter is a small unit of volume, one-thousandth of a liter. Common examples where you might encounter milliliters include:
- Liquid medications: Many liquid medicines are measured in milliliters.
- Cooking and baking: Recipes often call for specific volumes of liquids, such as milliliters of milk or water.
- Scientific experiments: Experiments frequently involve precise measurements of liquid volumes in milliliters.
- Beverages: Bottled water, soft drinks, and other beverages often have their volume specified in milliliters or liters.
Mass: How Much Matter Something Contains
mg (milligram) is a unit of mass. Mass is a measure of the amount of matter in an object. It's essentially how much "stuff" is present. A milligram is a very small unit of mass, one-thousandth of a gram. While mass and weight are often used interchangeably in everyday language, they are technically distinct. Mass remains constant regardless of location, while weight is affected by gravity. Milligrams are commonly used in contexts such as:
- Medicine: Dosage of medications is often specified in milligrams, indicating the amount of active ingredient.
- Nutrition labels: The amount of vitamins, minerals, and other nutrients in food is usually given in milligrams.
- Chemical experiments: Precise quantities of chemicals are often measured in milligrams for accuracy and consistency.
- Industrial processes: Manufacturing processes often require precise mass measurements in milligrams or grams.
The Crucial Difference: Why mL and mg Aren't Interchangeable
The fundamental difference between mL and mg is that they measure entirely different properties. You can't directly convert between them without knowing the density of the substance.
Density is the mass per unit volume of a substance. It tells you how much mass is packed into a given volume. The formula for density is:
Density = Mass / Volume
For example, the density of water is approximately 1 gram per milliliter (g/mL). This means that 1 mL of water has a mass of approximately 1 gram, or 1000 milligrams. However, the density of other substances varies greatly. A milliliter of oil will have a different mass (and therefore a different number of milligrams) than a milliliter of water. Similarly, a milliliter of mercury will have a much greater mass than a milliliter of water.
This is why you CANNOT simply equate mL and mg. Trying to do so can lead to dangerous errors, particularly in medical and chemical contexts.
Real-World Examples Illustrating the Difference
Let's illustrate the difference with some practical examples:
Example 1: Medication
A doctor prescribes 500 mg of a particular medication. This means the patient needs to take 500 milligrams of the active ingredient. If the medication is in liquid form, the volume needed (in mL) will depend entirely on the concentration of the medication, which is usually stated on the label. A higher concentration means a smaller volume is needed to deliver the required 500 mg.
Example 2: Baking a Cake
A recipe calls for 250 mL of milk. This refers to the volume of milk needed. The mass of that milk will depend on the density of the milk, which is close to, but not exactly, the same as water.
Example 3: Chemical Experiment
A chemistry experiment requires 100 mg of sodium chloride (table salt). The volume occupied by these 100 mg will depend on the density of the salt. If the salt is dissolved in water, the volume of the solution will depend on the concentration of the salt solution.
The Dangers of Confusing mL and mg
Confusing mL and mg can have serious consequences, particularly in the following scenarios:
- Medication errors: Administering the wrong volume of a medication can lead to underdosing (ineffective treatment) or overdosing (potentially fatal).
- Chemical accidents: Incorrectly measuring chemicals in a laboratory can result in chemical reactions with unintended consequences or dangerous levels of exposure.
- Misinterpretation of nutritional information: Confusing the volume and mass of food can lead to inaccurate estimations of caloric intake or nutrient consumption.
Practical Tips to Avoid Confusion
To avoid confusing mL and mg, always pay close attention to the units used in any measurement:
- Read labels carefully: When dealing with medications or chemicals, carefully read the labels to understand the units used for dosage or concentration.
- Consult professionals: If you are unsure about the proper dosage or measurement, always seek the advice of a healthcare professional or qualified chemist.
- Understand the context: The context in which the units are used should make it clear whether volume or mass is being measured.
- Remember density: Keep in mind that the relationship between mass and volume depends on density, which varies between substances.
Conclusion: mL and mg are Distinct and Crucial
In conclusion, mL and mg are distinct units that measure different properties – volume and mass, respectively. Understanding this fundamental difference is crucial for accuracy and safety, especially in the contexts of medicine, chemistry, and other scientific fields. Never interchange these units without considering the density of the substance being measured. Always carefully read labels, seek professional advice when needed, and maintain a sharp understanding of the context to prevent potentially harmful errors. By understanding the nuances of these units, you will significantly enhance your understanding of scientific principles and improve your overall safety.
Latest Posts
Latest Posts
-
Which Bond Is The Most Polar
Mar 17, 2025
-
Burning Of Candle Is Chemical Change
Mar 17, 2025
-
An Earth Satellite Moves In A Circular Orbit
Mar 17, 2025
-
What Is The Value Of K In Physics
Mar 17, 2025
-
The Study Of Tissues With A Microscope Is Called
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Ml And Mg . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
