What Is The Difference Between Density And Specific Gravity
News Leon
Mar 22, 2025 · 6 min read
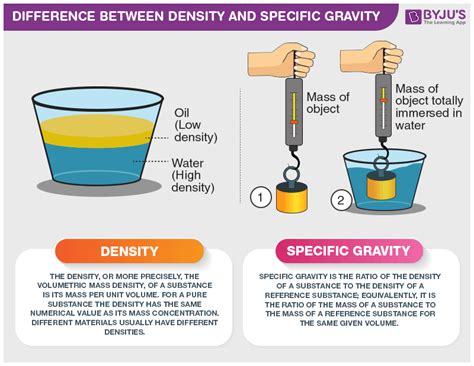
Table of Contents
Density vs. Specific Gravity: Understanding the Key Differences
Density and specific gravity are two closely related concepts often used in science, engineering, and everyday life. While they both describe the concentration of matter, they differ significantly in their definitions, units, and applications. Understanding these differences is crucial for accurate scientific measurements and interpretations. This comprehensive guide delves into the nuances of density and specific gravity, exploring their definitions, calculations, applications, and the key distinctions that set them apart.
What is Density?
Density is a fundamental property of matter that expresses the mass of a substance contained within a unit volume. In simpler terms, it describes how much "stuff" is packed into a given space. A higher density means more mass is crammed into the same volume, while a lower density indicates less mass for the same volume.
Formula for Density:
The density (ρ, pronounced "rho") of a substance is calculated using the following formula:
ρ = m / V
Where:
- ρ represents density (typically expressed in kg/m³ or g/cm³)
- m represents mass (typically expressed in kilograms or grams)
- V represents volume (typically expressed in cubic meters or cubic centimeters)
Units of Density:
The units of density depend on the units used for mass and volume. Common units include:
- kg/m³ (kilograms per cubic meter): The SI unit for density.
- g/cm³ (grams per cubic centimeter): A commonly used unit, especially in chemistry and materials science.
- g/mL (grams per milliliter): Equivalent to g/cm³, often used for liquids.
Understanding Specific Gravity
Specific gravity, also known as relative density, is a dimensionless quantity that compares the density of a substance to the density of a reference substance. This reference substance is typically water at 4°C (39.2°F), which has a density of approximately 1 g/cm³ or 1000 kg/m³. Therefore, specific gravity essentially tells you how many times denser or less dense a substance is compared to water.
Formula for Specific Gravity:
The specific gravity (SG) is calculated as:
SG = ρ<sub>substance</sub> / ρ<sub>water</sub>
Where:
- SG represents specific gravity (a dimensionless number)
- ρ<sub>substance</sub> represents the density of the substance
- ρ<sub>water</sub> represents the density of water at 4°C (approximately 1 g/cm³ or 1000 kg/m³)
Key Differences Between Density and Specific Gravity
The core difference lies in the nature of the measurement:
-
Density is an absolute measure of mass per unit volume. It's an intrinsic property of a substance, meaning it doesn't change depending on the amount of the substance you have. A kilogram of gold will always have the same density as a gram of gold.
-
Specific gravity is a relative measure comparing a substance's density to the density of water. It's a ratio, and therefore dimensionless. This makes it convenient for comparing the densities of different substances without needing to specify units.
Here's a table summarizing the key differences:
| Feature | Density | Specific Gravity |
|---|---|---|
| Definition | Mass per unit volume | Ratio of substance density to water density |
| Units | kg/m³, g/cm³, g/mL | Dimensionless |
| Nature | Absolute measure | Relative measure |
| Reference | None | Water at 4°C |
| Application | Material characterization, fluid dynamics | Comparing densities of different substances |
Applications of Density and Specific Gravity
Both density and specific gravity have numerous applications across various fields:
Applications of Density:
-
Material Identification: Density is a key property used to identify unknown materials. For example, a substance with a density of 19.3 g/cm³ is likely gold.
-
Fluid Mechanics: Density plays a critical role in understanding fluid flow, buoyancy, and pressure. For example, the density of air is crucial in aerodynamics.
-
Geophysics: Density measurements are used to study the Earth's interior and to locate underground resources such as oil and minerals.
-
Medicine: Body density measurements are used to estimate body fat percentage.
-
Manufacturing: Density is crucial in various manufacturing processes, ensuring the correct composition and consistency of products.
Applications of Specific Gravity:
-
Gemology: Specific gravity is a vital tool for identifying gemstones, as each gemstone has a characteristic specific gravity.
-
Mining and Metallurgy: Specific gravity is used to separate minerals based on their density differences. This is often done using techniques like flotation separation.
-
Food Industry: Specific gravity is used to determine the concentration of solids in liquids, such as in fruit juices or milk.
-
Chemical Engineering: Specific gravity is used for quality control and process optimization in various chemical processes.
-
Environmental Monitoring: Specific gravity measurements can help determine the concentration of pollutants in water samples.
Practical Examples and Calculations
Example 1: Calculating Density
Let's say we have a piece of metal with a mass of 50 grams and a volume of 5 cubic centimeters. To calculate its density:
ρ = m / V = 50 g / 5 cm³ = 10 g/cm³
Example 2: Calculating Specific Gravity
Suppose the same metal piece (from Example 1) has a density of 10 g/cm³. To calculate its specific gravity:
SG = ρ<sub>substance</sub> / ρ<sub>water</sub> = 10 g/cm³ / 1 g/cm³ = 10
This means the metal is 10 times denser than water.
Factors Affecting Density
Several factors can influence the density of a substance:
-
Temperature: Density typically decreases as temperature increases, as the molecules move further apart.
-
Pressure: Increased pressure generally leads to increased density, as the molecules are compressed closer together.
-
Phase: A substance's density can change dramatically depending on its phase (solid, liquid, or gas). Ice, for instance, is less dense than liquid water.
-
Composition: The density of a mixture or solution depends on the densities and proportions of its components.
Factors Affecting Specific Gravity
Since specific gravity is a ratio of densities, the factors affecting the density of the substance in question and the density of the reference substance (water) will influence the specific gravity. These include:
-
Temperature of both the substance and water: As mentioned earlier, temperature affects density. Therefore, variations in temperature of both the substance and water used as the reference will affect the measured specific gravity.
-
Pressure: Similar to density, pressure changes will impact the density of the substance and consequently its specific gravity. This is particularly relevant for gases.
-
Purity of the Substance: Impurities in the substance can alter its density and hence its specific gravity, leading to deviations from expected values.
Conclusion
Density and specific gravity are valuable tools for characterizing and comparing substances. While closely related, they represent distinct concepts – density being an absolute measure of mass per unit volume, and specific gravity being a relative measure comparing density to water. Understanding these differences is crucial for correctly interpreting measurements and applying them effectively in various scientific and engineering disciplines. The choice between using density or specific gravity often depends on the specific application and the type of information required. Both are essential tools in numerous fields, providing insights into the properties and behavior of matter.
Latest Posts
Latest Posts
-
What Are The Functions Of Areolar Tissue
Mar 22, 2025
-
A Wheelbarrow Is An Example Of Which Class Of Lever
Mar 22, 2025
-
Oxidation Number Of Cr In Cr2o72
Mar 22, 2025
-
Oxidation State Of N In Nh3
Mar 22, 2025
-
Capacity Of Doing Work Is Called
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Density And Specific Gravity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
