What Is The Conjugate Acid Of Nh2
News Leon
Mar 22, 2025 · 5 min read
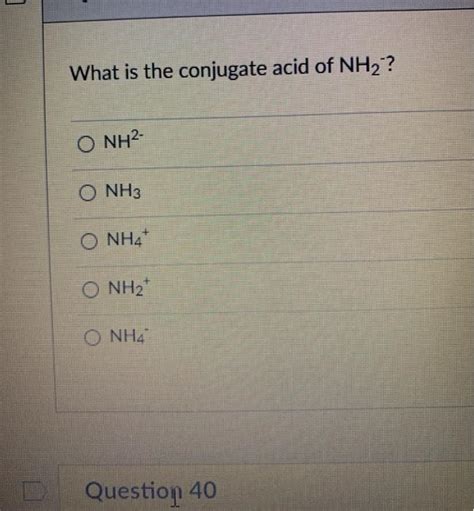
Table of Contents
What is the Conjugate Acid of NH₂⁻? A Deep Dive into Acid-Base Chemistry
Understanding conjugate acid-base pairs is fundamental to grasping acid-base chemistry. This article will delve deep into the concept, focusing specifically on the conjugate acid of the azanide ion (NH₂⁻). We'll explore its structure, properties, and reactions, offering a comprehensive understanding for students and anyone interested in learning more about this important chemical species.
Understanding Conjugate Acid-Base Pairs
Before we pinpoint the conjugate acid of NH₂⁻, let's establish the foundation. According to Brønsted-Lowry acid-base theory, an acid is a substance that donates a proton (H⁺), while a base is a substance that accepts a proton. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs are related by the difference of a single proton.
Think of it like a seesaw: the acid on one side and its conjugate base on the other, balanced by the proton transfer. This concept is crucial for understanding equilibrium reactions in aqueous solutions.
Key Takeaway: The conjugate acid of a base is formed by adding a proton (H⁺) to the base. The conjugate base of an acid is formed by removing a proton (H⁺) from the acid.
Identifying the Conjugate Acid of NH₂⁻
Now, let's focus on NH₂⁻, the azanide ion. This ion is a strong base, meaning it readily accepts protons. To find its conjugate acid, we simply add a proton (H⁺):
NH₂⁻ + H⁺ → NH₃
Therefore, the conjugate acid of NH₂⁻ is ammonia (NH₃).
Properties of Ammonia (NH₃)
Ammonia, the conjugate acid of NH₂⁻, is a colorless gas with a pungent, characteristic odor. It's highly soluble in water, forming an alkaline solution. Its properties are significantly different from its conjugate base, highlighting the dramatic transformation caused by protonation.
Physical Properties:
- Gas at room temperature: Ammonia exists as a gas under standard conditions.
- High solubility in water: It readily dissolves in water, forming ammonium hydroxide (NH₄OH), which is responsible for its alkaline nature.
- Sharp, pungent odor: Its distinctive smell is easily recognizable.
- Colorless: Pure ammonia gas is colorless.
Chemical Properties:
- Weak base: While its conjugate base, NH₂⁻, is a strong base, ammonia itself is a weak base. It only partially ionizes in water, meaning it doesn't completely dissociate into ions.
- Acts as a ligand: Ammonia can act as a ligand, forming coordination complexes with transition metal ions.
- Nucleophile: The nitrogen atom in ammonia possesses a lone pair of electrons, making it a good nucleophile, participating in nucleophilic substitution reactions.
- Reducing agent: Ammonia can act as a reducing agent, donating electrons in redox reactions.
Reactions Involving NH₂⁻ and NH₃
Understanding the reactions involving NH₂⁻ and its conjugate acid, NH₃, provides a deeper insight into their chemical behavior.
Reactions of NH₂⁻:
- Protonation: The most characteristic reaction of NH₂⁻ is its protonation to form ammonia (NH₃). This reaction is essentially instantaneous and irreversible in the presence of excess protons.
- Reactions with water: NH₂⁻ reacts vigorously with water, acting as a strong base and accepting a proton from water molecules. This reaction produces ammonia and hydroxide ions (OH⁻), contributing to the alkaline nature of the solution. The reaction is highly exothermic.
- Reactions with other acids: NH₂⁻ reacts readily with other acids, accepting protons and forming ammonia. The strength of the acid determines the extent and speed of the reaction.
Reactions of NH₃:
- Reaction with water: Ammonia acts as a weak base in water, partially accepting protons from water molecules to form ammonium ions (NH₄⁺) and hydroxide ions (OH⁻). This equilibrium is crucial in determining the pH of an ammonia solution.
- Reaction with acids: Ammonia readily reacts with acids, forming ammonium salts. For example, its reaction with hydrochloric acid (HCl) produces ammonium chloride (NH₄Cl).
- Formation of amides: Ammonia reacts with acyl chlorides to form amides, a significant reaction in organic chemistry.
The Importance of Understanding Conjugate Acid-Base Pairs
Understanding conjugate acid-base pairs is crucial for several reasons:
- Predicting reaction outcomes: Knowing the conjugate acid or base allows us to predict the outcome of acid-base reactions.
- Buffer solutions: Buffer solutions, crucial in maintaining a stable pH, are often composed of a weak acid and its conjugate base or a weak base and its conjugate acid.
- Understanding equilibrium constants: The equilibrium constant (Ka or Kb) for an acid-base reaction is directly related to the strength of the acid and its conjugate base.
- Analytical chemistry: Understanding conjugate acid-base pairs is essential for various analytical techniques, including titrations.
Applications of NH₃ and NH₂⁻
Both ammonia (NH₃) and the azanide ion (NH₂⁻) find various applications across different fields:
Applications of Ammonia (NH₃):
- Fertilizer production: Ammonia is a crucial component in the production of nitrogen-based fertilizers, vital for agriculture.
- Refrigerant: Ammonia's ability to absorb heat makes it a suitable refrigerant in industrial applications.
- Cleaning agent: Ammonia is a common ingredient in household cleaning products.
- Pharmaceutical industry: Ammonia is used in the synthesis of various pharmaceuticals.
- Textile industry: Ammonia is used in the production of synthetic fibers.
Applications of NH₂⁻ (though less direct due to its high reactivity):
- Synthesis of organic compounds: While NH₂⁻ itself is highly reactive and not directly used in many applications, its derivatives and related compounds are crucial intermediates in the synthesis of organic molecules. For instance, reactions involving amide formation often use reagents that generate intermediates related to the azanide ion.
- Research in materials science: Understanding the properties of the azanide ion is relevant to research focusing on new materials and their synthesis.
Conclusion
The azanide ion (NH₂⁻) is a strong base, and its conjugate acid is ammonia (NH₃), a weak base. Understanding the relationship between these two species, their properties, and their reactions is fundamental to comprehending acid-base chemistry. Both ammonia and the azanide ion, despite the high reactivity of the latter, play crucial roles in various applications, highlighting their importance in chemistry and related fields. This detailed exploration provides a comprehensive overview, enabling a deeper understanding of this critical acid-base pair. Further exploration into specific reaction mechanisms and applications can provide even more detailed insights into their unique chemical behaviors. The knowledge gained here serves as a solid foundation for more advanced studies in chemistry and related disciplines. Remember that always prioritize safety when handling chemicals, particularly strong bases like the azanide ion.
Latest Posts
Latest Posts
-
Anything That Has A Mass And Takes Up Space
Mar 23, 2025
-
Chromium Has An Atomic Mass Of 51 9961
Mar 23, 2025
-
A Meter Stick Balances Horizontally On A Knife Edge
Mar 23, 2025
-
Find The Lettered Angle In Each Case
Mar 23, 2025
-
Which Of The Following Reactions Does Not Involve Oxidation Reduction
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Acid Of Nh2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
