What Is The Conjugate Acid Of H2o
News Leon
Mar 18, 2025 · 5 min read
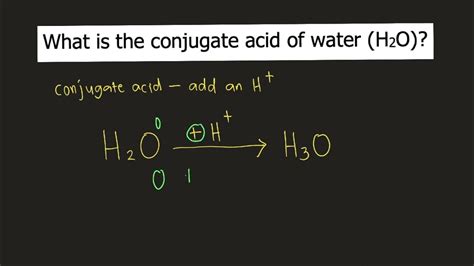
Table of Contents
What is the Conjugate Acid of H₂O? A Deep Dive into Acid-Base Chemistry
Understanding conjugate acid-base pairs is fundamental to grasping acid-base chemistry. This article delves deep into the concept, focusing specifically on the conjugate acid of water (H₂O). We'll explore the definition of conjugate acids and bases, examine the Brønsted-Lowry theory, and illustrate the behavior of water as both an acid and a base through various examples and practical applications.
Understanding Conjugate Acid-Base Pairs
According to the Brønsted-Lowry theory, an acid is a substance that donates a proton (H⁺), while a base is a substance that accepts a proton. Crucially, this theory defines conjugate pairs. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs are always related by the difference of a single proton (H⁺).
The Brønsted-Lowry Theory: A Cornerstone of Acid-Base Chemistry
The Brønsted-Lowry theory provides a comprehensive framework for understanding acid-base reactions. It's more encompassing than the Arrhenius theory, which limits acids to substances that produce H⁺ ions in aqueous solutions and bases to those producing OH⁻ ions. The Brønsted-Lowry theory expands the definition to include any proton donor (acid) and any proton acceptor (base), irrespective of the presence of water. This broader perspective allows us to analyze acid-base reactions in a wider range of solvents and situations.
Water: An Amphoteric Substance
Water (H₂O) exhibits a unique property: it's amphoteric. This means it can act as both an acid and a base. This dual nature is key to understanding its conjugate acid.
Water as an Acid
When water acts as an acid, it donates a proton (H⁺) to another substance. Consider the reaction of water with ammonia (NH₃):
H₂O + NH₃ ⇌ NH₄⁺ + OH⁻
In this reaction, water donates a proton to ammonia. Ammonia, acting as a base, accepts the proton, forming the ammonium ion (NH₄⁺). The hydroxide ion (OH⁻) is formed simultaneously. In this scenario, the conjugate base of water is the hydroxide ion (OH⁻).
Water as a Base
Conversely, water can act as a base, accepting a proton from another substance. Consider the reaction of water with hydrogen chloride (HCl):
HCl + H₂O ⇌ H₃O⁺ + Cl⁻
Here, water accepts a proton from hydrogen chloride. Hydrogen chloride, acting as an acid, donates the proton to water, forming the hydronium ion (H₃O⁺). The chloride ion (Cl⁻) is also produced. In this case, the conjugate acid of water is the hydronium ion (H₃O⁺).
The Conjugate Acid of H₂O: Hydronium Ion (H₃O⁺)
The conjugate acid of water is hydronium ion (H₃O⁺). This is formed when water accepts a proton from an acid. It's crucial to understand that while we often represent the proton as H⁺ in equations, in aqueous solutions, the proton is always associated with a water molecule, forming the hydronium ion. This is because a bare proton (H⁺) is extremely reactive and doesn't exist independently in solution.
The Importance of the Hydronium Ion in Acid-Base Chemistry
The hydronium ion plays a central role in defining the acidity of a solution. The pH scale, a measure of acidity or alkalinity, is directly related to the concentration of hydronium ions. A lower pH indicates a higher concentration of H₃O⁺ and thus a stronger acid.
Illustrative Examples: Exploring Conjugate Acid-Base Pairs involving Water
Let's explore some further examples to solidify our understanding:
Example 1: Reaction of Water with Acetic Acid
Acetic acid (CH₃COOH), a weak acid, reacts with water:
CH₃COOH + H₂O ⇌ CH₃COO⁻ + H₃O⁺
Here, acetic acid donates a proton to water, forming the acetate ion (CH₃COO⁻) and the hydronium ion (H₃O⁺). Water acts as a base, accepting the proton. Again, H₃O⁺ is the conjugate acid of H₂O. The acetate ion is the conjugate base of acetic acid.
Example 2: Reaction of Water with a Strong Acid like Hydrochloric Acid
A similar process occurs with strong acids like HCl:
HCl + H₂O → H₃O⁺ + Cl⁻
The reaction proceeds almost completely to the right because HCl is a strong acid, readily donating its proton to water. Once again, H₃O⁺ is the conjugate acid of H₂O.
Example 3: Autoionization of Water
Water can even react with itself in a process called autoionization:
2H₂O ⇌ H₃O⁺ + OH⁻
This equilibrium reaction shows that even pure water contains small but equal concentrations of H₃O⁺ and OH⁻ ions. This equilibrium constant (Kw) is a crucial aspect of understanding water's self-ionization properties and is a fundamental constant in many calculations related to pH.
Practical Applications and Significance
The concept of conjugate acids and bases, particularly the role of water and the hydronium ion, is vital in various fields:
- Environmental Science: Understanding the acidity of rainwater (acid rain) and its impact on ecosystems relies on the principles of conjugate acid-base reactions and the hydronium ion concentration.
- Medicine: Many biological processes rely on acid-base balance. Understanding buffer systems, which involve conjugate acid-base pairs, is crucial in maintaining physiological pH.
- Chemical Engineering: Many industrial processes involve acid-base reactions, and the concept of conjugate acids and bases is vital for optimizing reaction conditions and yields.
- Analytical Chemistry: Titration, a common analytical technique, directly uses acid-base reactions and understanding of conjugate pairs to determine the concentration of unknown solutions.
Conclusion: Mastering the Conjugate Acid of Water
Understanding the conjugate acid of water (H₃O⁺) is fundamental to mastering acid-base chemistry. Its formation and role in various reactions underscore the amphoteric nature of water and its significance in numerous scientific disciplines. By grasping these concepts and their practical applications, we can gain a deeper understanding of the intricate world of chemical reactions and their impact on our environment and technology. This knowledge allows for more precise predictions, more effective chemical processes, and a deeper comprehension of the natural world. Further exploration into buffer solutions, pH calculations, and various acid-base titration methods will further solidify this fundamental understanding. Therefore, continue your learning and master the subtleties of acid-base chemistry, which will be invaluable in many scientific and technological endeavors.
Latest Posts
Latest Posts
-
A Decrease In Demand And An Increase In Supply Will
Mar 19, 2025
-
Prove The Square Root Of 5 Is Irrational
Mar 19, 2025
-
The Figure Shown Is A Rectangle With A Semicircle
Mar 19, 2025
-
Letter To A Principal From Parent
Mar 19, 2025
-
The Amount Of Space Occupied By An Object
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Acid Of H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
