What Is The Bond Order Of Co
News Leon
Mar 24, 2025 · 6 min read
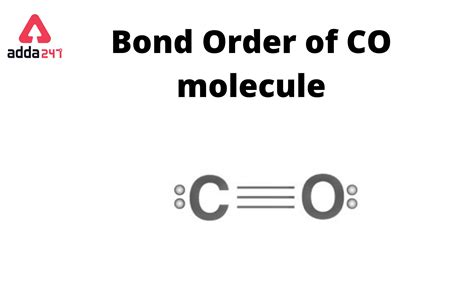
Table of Contents
What is the Bond Order of CO? A Deep Dive into Molecular Orbital Theory
The carbon monoxide molecule (CO) is a fascinating example of a stable diatomic molecule with a surprisingly strong triple bond. Understanding its bond order is crucial for comprehending its properties and reactivity. This article will delve into the molecular orbital theory (MOT) to explain how the bond order of CO is determined, exploring its implications for the molecule's stability and chemical behavior. We will also discuss the relationship between bond order and other molecular properties like bond length and bond energy.
Understanding Molecular Orbital Theory (MOT)
Before we calculate the bond order of CO, let's briefly review the fundamental principles of molecular orbital theory. MOT describes bonding in molecules by considering the combination of atomic orbitals to form molecular orbitals. These molecular orbitals encompass the entire molecule, not just individual atoms.
Key Concepts in MOT:
- Atomic Orbitals (AOs): These are regions of space around an atom where an electron is most likely to be found. Each atom contributes its valence electrons and their corresponding atomic orbitals to the formation of molecular orbitals.
- Molecular Orbitals (MOs): Formed by the linear combination of atomic orbitals (LCAO). These can be bonding orbitals (lower energy, stabilizing the molecule) or antibonding orbitals (higher energy, destabilizing the molecule).
- Bonding Orbitals: Electrons in bonding orbitals attract both nuclei, strengthening the bond between atoms.
- Antibonding Orbitals: Electrons in antibonding orbitals are located primarily outside the internuclear region, weakening the bond between atoms.
- Filling Molecular Orbitals: Electrons fill the molecular orbitals according to the Aufbau principle (lowest energy levels first), Hund's rule (maximizing unpaired electrons), and the Pauli exclusion principle (each orbital can hold a maximum of two electrons with opposite spins).
Determining the Bond Order of CO
Carbon has four valence electrons (2s²2p²) and oxygen has six valence electrons (2s²2p⁴). Therefore, the CO molecule has a total of 10 valence electrons to be distributed in its molecular orbitals.
The Molecular Orbital Diagram for CO:
The molecular orbital diagram for CO is constructed by combining the 2s and 2p atomic orbitals of carbon and oxygen. The order of energy levels is crucial and can vary slightly depending on the specific method used. However, a common representation shows the σ2s and σ2s orbitals lower in energy than the σ2p, π2p, π2p, and σ*2p orbitals.
-
σ2s and σ*2s orbitals: Two electrons fill the σ2s bonding orbital, and two electrons fill the σ*2s antibonding orbital. The net effect of these orbitals is zero on the bond order since the bonding and antibonding electrons cancel each other out.
-
σ2p orbital: Two electrons fill the σ2p bonding orbital, further strengthening the bond.
-
π2p orbitals: Four electrons fill the two degenerate π2p bonding orbitals. These π bonds contribute significantly to the bond strength.
-
Higher Energy Orbitals (π2p and σ2p): The higher energy antibonding orbitals, π2p and σ2p, remain unoccupied in the ground state of CO.
Calculating the Bond Order:
The bond order is calculated using the formula:
Bond Order = (Number of electrons in bonding orbitals - Number of electrons in antibonding orbitals) / 2
For CO:
- Number of electrons in bonding orbitals: 8 (2 in σ2s, 2 in σ2p, 4 in π2p)
- Number of electrons in antibonding orbitals: 2 (2 in σ*2s)
Bond Order = (8 - 2) / 2 = 3
Therefore, the bond order of CO is 3, indicating a triple bond between the carbon and oxygen atoms. This strong triple bond explains the high bond energy and short bond length observed in CO.
Implications of the Triple Bond in CO
The triple bond in CO has significant implications for its properties:
-
High Bond Energy: The strong triple bond results in a very high bond dissociation energy, meaning a large amount of energy is required to break the bond between carbon and oxygen. This contributes to the molecule's stability and low reactivity under normal conditions.
-
Short Bond Length: The triple bond pulls the carbon and oxygen atoms closer together, resulting in a shorter bond length compared to molecules with single or double bonds between the same atoms.
-
Limited Reactivity: The high bond energy and strong bond make CO relatively unreactive, although it can react under specific conditions with certain transition metals to form metal carbonyls.
-
Polarity: Despite being a triple bond, the bond in CO is polar due to the higher electronegativity of oxygen. This leads to a dipole moment, making CO a slightly polar molecule.
-
Spectroscopic Properties: The triple bond has a characteristic vibrational frequency that is detectable by infrared (IR) spectroscopy. This specific frequency is used to identify CO in various chemical environments.
Comparing CO to other Diatomic Molecules
Comparing CO to other diatomic molecules like N₂ (nitrogen) and O₂ (oxygen) helps to illustrate the relationship between bond order and molecular properties.
-
N₂: Nitrogen has a triple bond (bond order 3), similar to CO. This explains its high stability and inertness.
-
O₂: Oxygen has a double bond (bond order 2), making it more reactive than CO and N₂. Its paramagnetism also arises from the presence of two unpaired electrons in its π*2p antibonding orbitals.
The bond order directly relates to the strength and length of the bond, and influences the reactivity and other properties of the molecule.
Advanced Concepts and Considerations
While the simple MOT diagram provides a good understanding of CO's bond order, more sophisticated calculations can provide a more accurate picture. Density functional theory (DFT) and other computational methods offer refined descriptions of electron distribution and bonding in molecules like CO. These advanced methods account for electron correlation effects not explicitly considered in basic MOT.
Furthermore, the energy levels of molecular orbitals are influenced by factors such as interatomic distance and the presence of external electric or magnetic fields. These factors subtly affect the calculated bond order, making it important to always consider the context and the methodology used for its determination.
Conclusion
The bond order of CO is 3, signifying a strong triple bond. This triple bond dictates many of the fundamental properties of CO, including its high bond energy, short bond length, and relatively low reactivity. Understanding the molecular orbital diagram and applying the bond order calculation provides valuable insights into the structure, stability, and reactivity of this important molecule. This knowledge is crucial in various fields, from inorganic chemistry and materials science to atmospheric chemistry and astrochemistry, where CO plays significant roles. This deep dive into MOT highlights the power of theoretical chemistry in explaining and predicting the behavior of chemical compounds. By considering both simple models and more advanced computational techniques, we can achieve a more comprehensive understanding of chemical bonding and the properties of molecules like CO.
Latest Posts
Latest Posts
-
What Is The Opposite Of Hyperbole
Mar 26, 2025
-
Major And Minor Grooves In Dna
Mar 26, 2025
-
Tendons And Ligaments Are Examples Of
Mar 26, 2025
-
A Homogeneous Mixture Of Two Or More Substances
Mar 26, 2025
-
Reaction Of Ethanol And Acetic Acid
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Bond Order Of Co . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
