Reaction Of Ethanol And Acetic Acid
News Leon
Mar 26, 2025 · 6 min read
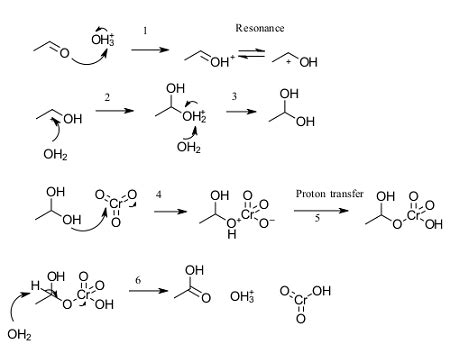
Table of Contents
The Reaction of Ethanol and Acetic Acid: Esterification Unveiled
The reaction between ethanol and acetic acid is a classic example of esterification, a crucial process in organic chemistry with wide-ranging applications in various industries. Understanding this reaction, its mechanism, factors influencing its rate and equilibrium, and its significance is crucial for anyone studying organic chemistry or involved in fields like pharmaceuticals, food science, and materials science. This comprehensive article will delve deep into the reaction between ethanol and acetic acid, exploring its intricacies and applications.
Understanding the Fundamentals: Esterification
Esterification is a reversible chemical reaction between a carboxylic acid and an alcohol to produce an ester and water. In the specific case of ethanol and acetic acid, the reaction produces ethyl acetate, a common ester with a fruity aroma, and water. The general reaction can be represented as:
RCOOH + R'OH ⇌ RCOOR' + H₂O
Where:
- RCOOH represents the carboxylic acid (in this case, acetic acid, CH₃COOH)
- R'OH represents the alcohol (in this case, ethanol, CH₃CH₂OH)
- RCOOR' represents the ester (in this case, ethyl acetate, CH₃COOCH₂CH₃)
- H₂O represents water
This reaction is an equilibrium reaction, meaning it proceeds in both the forward (esterification) and reverse (hydrolysis) directions. The position of equilibrium depends on several factors, which we'll explore later.
The Mechanism of Esterification: A Step-by-Step Approach
The esterification reaction doesn't occur in a single step. Instead, it involves a series of steps that can be summarized as follows:
-
Protonation of the carbonyl oxygen: The carbonyl oxygen of the carboxylic acid (acetic acid) is protonated by a strong acid catalyst, typically sulfuric acid (H₂SO₄). This protonation increases the electrophilicity of the carbonyl carbon, making it more susceptible to nucleophilic attack.
-
Nucleophilic attack by the alcohol: The oxygen atom of the alcohol (ethanol) acts as a nucleophile, attacking the electrophilic carbonyl carbon. This forms a tetrahedral intermediate.
-
Proton transfer: A proton is transferred from the hydroxyl group of the tetrahedral intermediate to one of the hydroxyl groups attached to the carbonyl carbon.
-
Elimination of water: A water molecule is eliminated from the tetrahedral intermediate, leading to the formation of the ester (ethyl acetate).
-
Deprotonation: The protonated ester is deprotonated, regenerating the acid catalyst.
This mechanism highlights the importance of the acid catalyst in facilitating the reaction. The acid catalyst protonates the carbonyl oxygen, making it a better electrophile and accelerating the reaction.
Factors Affecting the Esterification Reaction
Several factors significantly influence the rate and equilibrium position of the esterification reaction between ethanol and acetic acid:
1. Temperature:
Increasing the temperature generally increases the rate of the reaction. Higher temperatures provide more kinetic energy to the molecules, leading to more frequent and effective collisions, thus accelerating the reaction rate. However, excessively high temperatures can lead to undesirable side reactions.
2. Concentration of Reactants:
The rate of the reaction increases with an increase in the concentration of either ethanol or acetic acid. Higher concentrations lead to more frequent collisions between the reactant molecules, increasing the likelihood of successful reactions.
3. Concentration of Catalyst:
The acid catalyst, usually sulfuric acid, plays a crucial role. Increasing the concentration of the catalyst increases the reaction rate by increasing the protonation of the carbonyl oxygen. However, excessively high concentrations may lead to side reactions or catalyst poisoning.
4. Water Content:
Since esterification is a reversible reaction, the presence of water shifts the equilibrium towards the reactants (ethanol and acetic acid). Removing water from the reaction mixture, often through techniques like azeotropic distillation, helps drive the reaction forward, increasing the yield of ethyl acetate.
5. Choice of Catalyst:
While sulfuric acid is a common catalyst, other strong acids can also be used. The choice of catalyst can influence the reaction rate and selectivity, depending on its strength and potential for side reactions.
Applications of Ethyl Acetate
Ethyl acetate, the product of the reaction between ethanol and acetic acid, finds extensive applications across various industries:
1. Solvent:
Ethyl acetate is a widely used solvent in various industrial processes, including:
- Coating industries: It's a solvent in paints, lacquers, and varnishes, facilitating the even distribution of the coating material.
- Pharmaceutical industry: Used in the production of pharmaceuticals as a solvent for extracting active ingredients and in the formulation of medicines.
- Cleaning agents: Found in nail polish removers, adhesives, and other cleaning solutions due to its ability to dissolve many organic substances.
- Food industry: Used as a food additive in certain products due to its fruity aroma.
2. Flavoring Agent:
The fruity aroma of ethyl acetate makes it a valuable flavoring agent in many food products, contributing to the characteristic taste and smell of fruits like bananas and pineapples. It's often used in artificial flavorings and perfumes.
3. Chemical Intermediate:
Ethyl acetate serves as a crucial chemical intermediate in the production of other chemicals. Its reactivity allows for further modifications to synthesize more complex molecules.
4. Extraction Solvent:
Due to its ability to dissolve many organic substances, ethyl acetate is used as an extraction solvent in various separation processes.
Optimization of the Esterification Reaction: Yield and Efficiency
Maximizing the yield of ethyl acetate requires careful control of the reaction parameters. Here are some strategies for optimizing the esterification:
- Careful selection of catalyst concentration: Finding the optimal concentration that balances the rate enhancement with the potential for side reactions is crucial.
- Efficient water removal: Techniques like azeotropic distillation, which uses a co-boiling agent to remove water, significantly improve the yield by shifting the equilibrium towards the product.
- Control of reaction temperature: Maintaining the optimal temperature is essential to avoid side reactions and maximize reaction efficiency.
- Use of excess reactants: Using an excess of one reactant can drive the equilibrium towards the product, increasing the yield.
Conclusion: A Versatile Reaction with Broad Significance
The reaction between ethanol and acetic acid is more than just a textbook example of esterification. It's a fundamental process with far-reaching applications in many industries. Understanding the reaction mechanism, factors affecting its equilibrium, and methods for optimizing its yield is crucial for anyone working in fields involving organic synthesis or the use of ethyl acetate. The fruity aroma of ethyl acetate, a product of this reaction, serves as a testament to the versatility and importance of this seemingly simple chemical transformation. From the laboratory to large-scale industrial production, the esterification of ethanol and acetic acid remains a significant and widely applied reaction in the world of chemistry. Further research continues to explore innovative ways to improve its efficiency and expand its applications, solidifying its position as a cornerstone of organic chemistry and industrial processes. By mastering the intricacies of this reaction, we unlock possibilities for innovation and development across numerous scientific and technological fields.
Latest Posts
Latest Posts
-
What Is 0 07 As A Percentage
Mar 29, 2025
-
How Many Unpaired Electrons Does Oxygen Have
Mar 29, 2025
-
What Is Not True About The Cell Membrane
Mar 29, 2025
-
Example Of Request Letter For Permission
Mar 29, 2025
-
Largest Foramen In The Human Skeleton
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Reaction Of Ethanol And Acetic Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
