How Many Unpaired Electrons Does Oxygen Have
News Leon
Mar 29, 2025 · 5 min read
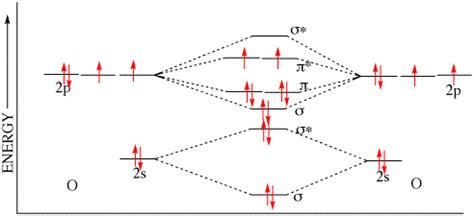
Table of Contents
How Many Unpaired Electrons Does Oxygen Have? A Deep Dive into Atomic Structure and Electron Configuration
Oxygen, the life-giving element, plays a crucial role in our existence. Understanding its atomic structure, particularly the number of unpaired electrons, is key to comprehending its chemical reactivity and the formation of crucial molecules like water. This article delves deep into the electronic configuration of oxygen, explaining how to determine the number of unpaired electrons and exploring the implications of this characteristic.
Understanding Electron Configuration
Before we tackle the number of unpaired electrons in oxygen, let's establish a foundational understanding of electron configuration. This describes the arrangement of electrons in the different energy levels and sublevels within an atom. Electrons occupy orbitals, regions of space where there's a high probability of finding an electron. Each orbital can hold a maximum of two electrons, and these electrons must have opposite spins (represented as ↑ and ↓).
The filling of orbitals follows specific rules:
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy level and moving upwards.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms). This means each orbital can hold a maximum of two electrons with opposite spins.
- Hund's Rule: Within a subshell (like p or d), electrons will individually occupy each orbital before pairing up in any one orbital. This minimizes electron-electron repulsion.
Determining Oxygen's Electron Configuration
Oxygen (O) has an atomic number of 8, meaning it possesses 8 protons and 8 electrons in a neutral atom. To determine its electron configuration, we follow the Aufbau principle:
-
The first energy level (n=1) contains the 1s subshell: This subshell can hold a maximum of two electrons. Oxygen fills this subshell completely: 1s².
-
The second energy level (n=2) contains the 2s and 2p subshells: The 2s subshell holds two electrons (2s²). The 2p subshell has three orbitals, each capable of holding two electrons, for a total of six electrons. Oxygen has six electrons remaining to fill this subshell.
-
Filling the 2p subshell: According to Hund's Rule, we fill each 2p orbital individually before pairing electrons. This results in the configuration: 2pₓ¹ 2pᵧ¹ 2pᶻ².
Therefore, the complete electron configuration of oxygen is 1s²2s²2p⁴.
Counting Unpaired Electrons in Oxygen
Now, let's count the unpaired electrons. Looking at the 2p subshell configuration (2pₓ¹ 2pᵧ¹ 2pᶻ²), we see that two of the 2p orbitals are singly occupied (2pₓ and 2pᵧ), while one is doubly occupied (2pᶻ). This means oxygen has two unpaired electrons.
The Significance of Unpaired Electrons in Oxygen's Reactivity
The presence of two unpaired electrons is crucial to oxygen's high reactivity. These unpaired electrons readily participate in chemical bonding, forming covalent bonds with other atoms. This ability to form bonds explains oxygen's critical role in numerous chemical reactions, including:
- Combustion: Oxygen readily reacts with fuels, releasing a large amount of energy in the process. This reaction involves the pairing of oxygen's unpaired electrons with electrons from the fuel.
- Respiration: Oxygen is essential for cellular respiration, the process by which organisms convert energy from food. This process involves the transfer of electrons to oxygen, forming water and releasing energy.
- Oxidation: Oxygen is a potent oxidizing agent, meaning it readily accepts electrons from other substances. This process, known as oxidation, is fundamental to many chemical and biological processes, including rusting and the breakdown of organic matter.
Orbital Diagrams and Visualizing Unpaired Electrons
Visualizing the electron configuration using orbital diagrams can further clarify the number of unpaired electrons. Each orbital is represented by a box, and electrons are shown as arrows.
For oxygen:
- 1s: ↑↓
- 2s: ↑↓
- 2pₓ: ↑
- 2pᵧ: ↑
- 2pᶻ: ↑↓
The diagram clearly shows the two unpaired electrons in the 2p subshell.
Oxygen's Role in Biological Systems and its Unpaired Electrons
The reactivity stemming from oxygen's unpaired electrons is directly responsible for its vital role in biological systems. The formation of water (H₂O), a crucial solvent and participant in countless biological processes, is a direct consequence of oxygen's ability to form two covalent bonds. Similarly, the incorporation of oxygen into organic molecules through respiration is pivotal for energy production in living organisms.
The very air we breathe is a testament to oxygen’s significance. Without its unique electronic configuration and its resulting reactivity, life as we know it would be impossible. Understanding the fundamental nature of oxygen's unpaired electrons provides crucial insight into this remarkable element's impact on our world.
Comparing Oxygen's Electron Configuration to Other Elements
Comparing oxygen's electron configuration to neighboring elements on the periodic table helps to further solidify our understanding. For instance, nitrogen (N) with seven electrons has three unpaired electrons in its 2p subshell. Fluorine (F), with nine electrons, has only one unpaired electron, as its 2p subshell is almost filled. This variation in unpaired electrons directly impacts the reactivity and bonding properties of each element.
Advanced Concepts: Paramagnetism and Oxygen
The presence of unpaired electrons also leads to a phenomenon known as paramagnetism. Paramagnetic substances are weakly attracted to magnetic fields. This is because the unpaired electrons generate a small magnetic moment. Oxygen's paramagnetism is a direct consequence of its two unpaired electrons. This property can be experimentally verified using a magnetic susceptibility balance, demonstrating the practical implications of the electronic configuration.
Conclusion: The Importance of Understanding Unpaired Electrons
In conclusion, oxygen possesses two unpaired electrons due to its electronic configuration of 1s²2s²2p⁴. This seemingly small detail significantly impacts its chemical behavior and biological importance. The unpaired electrons drive oxygen’s high reactivity, enabling it to form crucial bonds, participate in vital processes like respiration and combustion, and maintain the delicate balance of life on Earth. Understanding the relationship between electron configuration, unpaired electrons, and chemical reactivity is paramount in various fields, from chemistry and biology to materials science and environmental studies. The simple yet powerful principle of unpaired electrons underscores the beauty and complexity of atomic structure and its impact on the world around us.
Latest Posts
Latest Posts
-
Why Is Bone Considered Connective Tissue
Mar 31, 2025
-
One Billion Equal To How Many Crores
Mar 31, 2025
-
If The Cross Product Of Two Vectors Is Zero
Mar 31, 2025
-
How To Use Insert In Python
Mar 31, 2025
-
How Many Lines Of Symmetry Does Scalene Triangle Have
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Unpaired Electrons Does Oxygen Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
