A Homogeneous Mixture Of Two Or More Substances
News Leon
Mar 26, 2025 · 6 min read
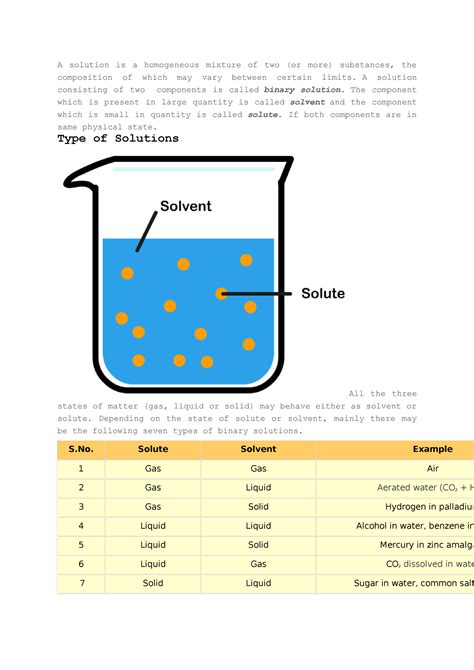
Table of Contents
A Homogeneous Mixture: A Deep Dive into Uniformity
A homogeneous mixture, in the simplest terms, is a substance where two or more components are combined and are uniformly distributed throughout the mixture. Unlike heterogeneous mixtures, which show visible differences in composition, homogeneous mixtures present a consistent appearance and properties throughout the sample. This uniformity is key to understanding their behavior and applications across various fields of science and everyday life. This article will delve into the characteristics, types, examples, and significance of homogeneous mixtures.
Understanding the Fundamentals: Defining Homogeneity
The defining characteristic of a homogeneous mixture is its uniformity. This means that no matter where you take a sample from the mixture, its composition will remain the same. This is in stark contrast to a heterogeneous mixture, where different parts of the mixture will have different compositions. For example, a salad is a heterogeneous mixture because you can easily see and separate the different ingredients (lettuce, tomatoes, cucumbers, etc.). Conversely, saltwater is a homogeneous mixture because the salt is evenly dissolved in the water, and you can't visually distinguish the salt from the water.
Key Properties of Homogeneous Mixtures
- Uniform Composition: The most crucial feature, as mentioned earlier. This uniformity extends to the macroscopic (visible) and microscopic levels.
- Single Phase: Homogeneous mixtures exist in a single phase, whether solid, liquid, or gas. You won't see distinct layers or regions of different properties.
- Invisible Components: The individual components of a homogeneous mixture are not visible to the naked eye. They are dispersed at the molecular or ionic level.
- Difficult Separation: Separating the components of a homogeneous mixture requires more sophisticated techniques than simply picking out different pieces, like filtration or distillation.
Types of Homogeneous Mixtures: Exploring the Diversity
Homogeneous mixtures can be classified based on the state of matter of the components:
1. Solid Solutions (Alloys):
These mixtures consist of two or more solid components blended uniformly. A classic example is alloys, which are mixtures of metals. Steel, for instance, is an alloy of iron and carbon, while brass is an alloy of copper and zinc. The properties of alloys often differ significantly from their constituent metals, allowing for the creation of materials with enhanced strength, durability, or other desirable characteristics. The even distribution of the components at the atomic level contributes to the consistent properties of these solid solutions.
Examples of Solid Solutions:
- Steel: Iron and carbon
- Brass: Copper and zinc
- Bronze: Copper and tin
- 14k Gold: Gold and other metals (like copper and silver)
2. Liquid Solutions:
These are arguably the most common type of homogeneous mixture. They involve one or more substances dissolved in a liquid solvent. The solvent is the component present in the largest amount, while the dissolved substances are called solutes. The solutes are distributed uniformly at the molecular level throughout the solvent, leading to a homogenous appearance.
Examples of Liquid Solutions:
- Saltwater: Salt (solute) dissolved in water (solvent)
- Sugar water: Sugar (solute) dissolved in water (solvent)
- Air (partially): While air is a mixture of gases, many consider the combination of oxygen and nitrogen in air to be a homogeneous liquid solution when considering the small amount of water vapor present in air.
- Vinegar: Acetic acid (solute) dissolved in water (solvent)
- Many drinks: Soda, juice, etc. (often with various solutes like sugars, acids, and flavorings dissolved in water).
3. Gaseous Solutions:
These mixtures comprise two or more gases uniformly mixed. The gases are distributed evenly throughout the mixture, making it homogeneous. Air is a prime example, being a mixture of primarily nitrogen, oxygen, argon, and trace amounts of other gases. The gases are uniformly dispersed, making it a homogenous gaseous solution.
Examples of Gaseous Solutions:
- Air: Nitrogen, oxygen, argon, carbon dioxide, and other trace gases
- Natural gas: Primarily methane, with smaller amounts of ethane, propane, and other hydrocarbons.
- Diving gas: Mixtures of oxygen and other gases like helium or nitrogen, adjusted for different depths.
Methods for Separating Components of Homogeneous Mixtures
Unlike heterogeneous mixtures, separating the components of a homogeneous mixture requires more advanced techniques because the components are intimately mixed at a molecular or ionic level. Several methods are used, depending on the nature of the mixture:
- Distillation: This technique separates components based on their boiling points. The mixture is heated, and the component with the lower boiling point vaporizes first, then condenses separately. This is commonly used to separate liquids with different boiling points, such as alcohol and water.
- Evaporation: This method is suitable for separating a dissolved solid from a liquid solvent. The liquid is heated, and the solvent evaporates, leaving behind the solid residue. The process is commonly used to obtain salt from saltwater.
- Chromatography: This technique separates components based on their differing affinities for a stationary and a mobile phase. The mixture is passed through a column or a paper strip, and the components separate based on their interactions with the stationary and mobile phases. This technique is highly versatile and used in many scientific applications.
- Crystallization: This technique relies on the differences in solubility of the components at different temperatures. The solution is slowly cooled or the solvent is evaporated, leading to the formation of crystals of the solute.
- Filtration (for some specific cases): In certain instances, where a homogeneous mixture undergoes a change, filtration may become a viable method. For example, if a homogeneous solution of a solid and liquid creates a precipitate, the solid can be separated from the liquid using filtration. However, it's crucial that a solid phase forms for this method to work.
The Significance of Homogeneous Mixtures
Homogeneous mixtures play a crucial role in various aspects of our lives and scientific endeavors. Their consistent composition and properties make them essential in many applications:
- Industrial Processes: Many industrial processes rely on homogeneous mixtures. The production of alloys, the synthesis of chemicals, and the preparation of pharmaceutical formulations all involve homogeneous mixtures.
- Food and Beverage Industry: The food and beverage industry extensively uses homogeneous mixtures. Many beverages, sauces, and processed foods are homogeneous mixtures designed for consistent flavor and texture.
- Biological Systems: Many biological systems rely on homogeneous mixtures. Blood, for example, is a complex homogeneous mixture that transports oxygen, nutrients, and waste products throughout the body.
- Environmental Science: The study of air and water pollution often involves analyzing homogeneous mixtures to determine the concentration of pollutants.
- Medical Applications: Many medical solutions, such as intravenous fluids and medications, are homogeneous mixtures designed to provide a consistent delivery of active substances.
Conclusion: The Ubiquity of Homogeneous Mixtures
Homogeneous mixtures are ubiquitous in our world, appearing in everything from the air we breathe to the materials used to build our homes. Understanding their characteristics, types, and methods of separation is crucial in various scientific and industrial contexts. The consistent properties and uniform composition of these mixtures make them essential in numerous applications, highlighting their importance in our daily lives and technological advancements. Further research and exploration of homogeneous mixtures continue to unlock new possibilities and innovations across various disciplines.
Latest Posts
Latest Posts
-
How Many Unpaired Electrons Does Oxygen Have
Mar 29, 2025
-
What Is Not True About The Cell Membrane
Mar 29, 2025
-
Example Of Request Letter For Permission
Mar 29, 2025
-
Largest Foramen In The Human Skeleton
Mar 29, 2025
-
In Humans The Diploid Number Of Chromosomes Is
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about A Homogeneous Mixture Of Two Or More Substances . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
