What Is Molar Mass Of Iron
News Leon
Mar 19, 2025 · 5 min read
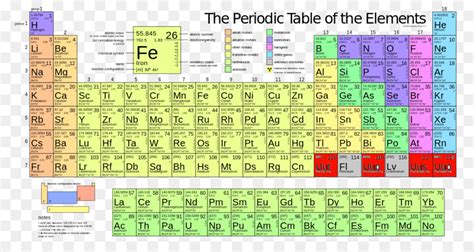
Table of Contents
What is the Molar Mass of Iron? A Deep Dive into Atomic Weight and its Applications
Iron, a ubiquitous element essential to life and industry, plays a pivotal role in numerous applications. Understanding its properties, especially its molar mass, is fundamental to various scientific and engineering fields. This comprehensive guide delves into the intricacies of iron's molar mass, explaining its calculation, significance, and practical applications.
Understanding Molar Mass: The Foundation
Before focusing specifically on iron, let's establish a clear understanding of molar mass. Molar mass, also known as molecular weight, represents the mass of one mole of a substance. A mole is a fundamental unit in chemistry, defined as the amount of a substance containing Avogadro's number (approximately 6.022 x 10<sup>23</sup>) of constituent particles (atoms, molecules, ions, etc.). Therefore, the molar mass essentially tells us the mass of 6.022 x 10<sup>23</sup> atoms, molecules, or other entities of a particular substance.
The molar mass is typically expressed in grams per mole (g/mol). It's crucial to distinguish between molar mass and atomic mass. Atomic mass represents the mass of a single atom, while molar mass represents the mass of a mole of atoms. The numerical value of both is the same, but the units differ.
Calculating the Molar Mass of Iron (Fe)
Iron's molar mass is determined by its average atomic mass, which is a weighted average of the masses of all naturally occurring isotopes of iron. Iron has four stable isotopes: <sup>54</sup>Fe, <sup>56</sup>Fe, <sup>57</sup>Fe, and <sup>58</sup>Fe. Each isotope contributes to the overall average atomic mass based on its natural abundance.
The most abundant isotope of iron is <sup>56</sup>Fe, accounting for approximately 91.75% of naturally occurring iron. The other isotopes have significantly lower abundances. To calculate the average atomic mass, we weigh each isotope's mass by its abundance:
- <sup>54</sup>Fe: Atomic mass ≈ 53.940 amu, Abundance ≈ 5.85%
- <sup>56</sup>Fe: Atomic mass ≈ 55.935 amu, Abundance ≈ 91.75%
- <sup>57</sup>Fe: Atomic mass ≈ 56.935 amu, Abundance ≈ 2.12%
- <sup>58</sup>Fe: Atomic mass ≈ 57.933 amu, Abundance ≈ 0.28%
Calculating the weighted average:
(53.940 amu * 0.0585) + (55.935 amu * 0.9175) + (56.935 amu * 0.0212) + (57.933 amu * 0.0028) ≈ 55.845 amu
Therefore, the average atomic mass of iron is approximately 55.845 atomic mass units (amu). Since 1 amu is approximately equal to 1 gram per mole (g/mol), the molar mass of iron is approximately 55.845 g/mol. This value is widely accepted and used in various chemical calculations.
Significance of Iron's Molar Mass
Knowing the molar mass of iron is paramount in several crucial applications:
1. Stoichiometric Calculations:
Molar mass is a cornerstone of stoichiometry, allowing us to convert between mass and moles of iron in chemical reactions. For instance, if we need to determine the mass of iron required for a specific chemical reaction, we can use its molar mass as a conversion factor. This precise calculation ensures accurate reactant proportions for desired reaction outcomes.
2. Determining the Number of Atoms:
Using Avogadro's number and the molar mass, we can determine the precise number of iron atoms present in a given mass of iron. This is crucial in fields like materials science where the number of atoms can significantly influence material properties.
3. Concentration Calculations:
In solutions involving iron compounds, molar mass is essential for calculating the concentration of iron ions in moles per liter (Molarity). This is critical in analytical chemistry and various industrial processes where precise concentration control is required.
4. Material Science and Engineering:
Iron's molar mass is critical in calculating the composition and properties of iron-based alloys and steels. Precise molar mass knowledge is essential for designing materials with specific mechanical, electrical, and magnetic characteristics.
5. Geochemical Studies:
In geology and geochemistry, iron's molar mass aids in quantifying the concentration of iron in rocks and minerals, providing insights into geological processes and the Earth's composition.
Applications of Iron and its Molar Mass in Different Fields
The applications of iron and the knowledge of its molar mass are widespread and impact various sectors:
1. Metallurgy and Steel Production:
Iron is the primary component of steel, a fundamental material in construction, manufacturing, and transportation. Molar mass calculations are crucial in controlling the alloying proportions of steel, ensuring desired strength, hardness, and other mechanical properties.
2. Biochemistry and Medicine:
Iron is an essential element in biological systems, forming part of hemoglobin, which transports oxygen in blood. Understanding iron's molar mass helps in assessing iron levels in the body and diagnosing conditions like anemia. Furthermore, iron-containing compounds are used in various medical applications, including MRI contrast agents.
3. Catalysis:
Iron-based catalysts are used in numerous industrial processes, including the Haber-Bosch process for ammonia synthesis. Accurate calculations involving iron's molar mass are essential for optimizing catalyst performance and maximizing reaction yields.
4. Environmental Science:
Iron plays a crucial role in environmental processes, including nutrient cycling and contaminant remediation. Understanding its molar mass aids in modeling its behavior in different environments and assessing its impact on ecosystems.
5. Electrochemistry:
Iron's electrochemical properties and its molar mass are critical in designing and optimizing electrochemical processes such as corrosion prevention and battery technologies.
Beyond the Basics: Isotopic Variations and Their Impact
While we've used the average molar mass of iron, it's essential to recognize that this is an average value. The exact molar mass can slightly vary depending on the isotopic composition of the iron sample. This variation, though often minor, becomes significant in high-precision scientific measurements and specialized applications.
Conclusion: The Importance of Precision
The molar mass of iron, while seemingly a simple concept, is a critical parameter in a vast array of scientific, engineering, and industrial applications. Accurate calculation and understanding of this value are essential for precise stoichiometric calculations, material characterization, and process optimization across diverse fields. From the intricate workings of the human body to the construction of towering skyscrapers, iron and its molar mass are fundamental building blocks of our modern world. The precision inherent in understanding its molar mass ensures accuracy and efficiency in countless applications.
Latest Posts
Latest Posts
-
Which Of The Following Is An Even Function
Mar 20, 2025
-
Four Capacitors Are Connected As Shown In The Figure
Mar 20, 2025
-
What Energy Tranformation Happens In A Motot
Mar 20, 2025
-
Which Of The Following Is A Nonessential Amino Acid
Mar 20, 2025
-
In Humans Free Earlobes Are Dominant To Attached Earlobes
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Is Molar Mass Of Iron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
