What Is Conjugate Base Of Hso4
News Leon
Mar 25, 2025 · 5 min read
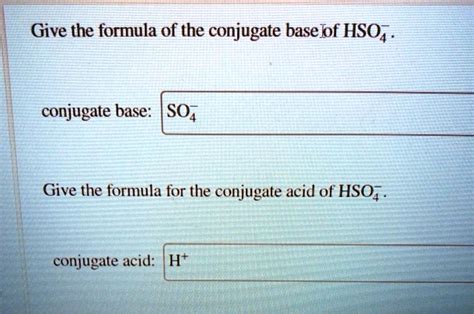
Table of Contents
What is the Conjugate Base of HSO₄⁻? Understanding Acids, Bases, and Conjugate Pairs
The question, "What is the conjugate base of HSO₄⁻?" delves into the fundamental concepts of acid-base chemistry. Understanding conjugate acid-base pairs is crucial for grasping equilibrium reactions, pH calculations, and buffer solutions. This comprehensive guide will not only answer this specific question but also provide a solid foundation in acid-base theory.
Understanding Acids and Bases
Before diving into conjugate bases, let's refresh our understanding of acids and bases. Several definitions exist, but the most relevant for this discussion is the Brønsted-Lowry definition.
Brønsted-Lowry Theory: This theory defines an acid as a substance that donates a proton (H⁺), and a base as a substance that accepts a proton. This proton transfer is the central feature of acid-base reactions.
Key Characteristics of Acids and Bases:
- Acids: Typically have a sour taste, react with metals to produce hydrogen gas, and change the color of certain indicators (like litmus paper to red). They often contain hydrogen atoms that can be donated as protons.
- Bases: Typically have a bitter taste, feel slippery, and change the color of indicators (like litmus paper to blue). They often contain hydroxide ions (OH⁻) or other groups capable of accepting protons.
Conjugate Acid-Base Pairs: A Definition
A conjugate acid-base pair consists of two species that differ by a single proton (H⁺). When an acid donates a proton, the remaining species is its conjugate base. Conversely, when a base accepts a proton, the resulting species is its conjugate acid.
The relationship is reciprocal: the conjugate base can act as a base by accepting a proton to reform the original acid, and the conjugate acid can act as an acid by donating a proton to reform the original base.
Determining the Conjugate Base of HSO₄⁻
Now, let's address the central question: What is the conjugate base of HSO₄⁻ (hydrogen sulfate ion)?
HSO₄⁻ acts as an acid by donating a proton (H⁺). When it does so, it loses one hydrogen ion, leaving behind SO₄²⁻.
Therefore, the conjugate base of HSO₄⁻ is SO₄²⁻ (sulfate ion).
Illustrative Example: The Reaction of HSO₄⁻ with Water
Consider the reaction of HSO₄⁻ with water:
HSO₄⁻(aq) + H₂O(l) ⇌ H₃O⁺(aq) + SO₄²⁻(aq)
In this reaction:
- HSO₄⁻ acts as an acid, donating a proton to water.
- H₂O acts as a base, accepting the proton from HSO₄⁻.
- H₃O⁺ (hydronium ion) is the conjugate acid of H₂O.
- SO₄²⁻ (sulfate ion) is the conjugate base of HSO₄⁻.
This equilibrium demonstrates the reversible nature of acid-base reactions and the interplay between conjugate pairs.
Amphoteric Nature of HSO₄⁻
It's important to note that HSO₄⁻ exhibits amphoteric behavior. This means it can act as both an acid and a base, depending on the reaction conditions.
- As an acid: As shown above, it donates a proton to a stronger base like water.
- As a base: It can accept a proton from a stronger acid. For instance, in the presence of a strong acid like sulfuric acid (H₂SO₄), HSO₄⁻ can act as a base:
H₂SO₄(aq) + HSO₄⁻(aq) ⇌ H₃SO₄⁺(aq) + SO₄²⁻(aq)
The Importance of Conjugate Bases in Acid-Base Chemistry
Understanding conjugate bases is essential for several reasons:
- Predicting reaction outcomes: Knowing the conjugate base allows us to predict the products of acid-base reactions.
- Calculating pH: The strength of a conjugate base influences the pH of a solution. Strong acids have weak conjugate bases, and weak acids have stronger conjugate bases.
- Buffer solutions: Buffer solutions, which resist changes in pH, are formed from a weak acid and its conjugate base (or a weak base and its conjugate acid). The conjugate base plays a crucial role in neutralizing added acids.
- Understanding equilibrium: Conjugate acid-base pairs are key to understanding equilibrium constants (Ka and Kb) in acid-base reactions.
Factors Affecting Conjugate Base Strength
The strength of a conjugate base is inversely proportional to the strength of its corresponding acid.
- Strong acids have weak conjugate bases: Strong acids completely dissociate in water, leaving behind a conjugate base that has minimal tendency to accept a proton.
- Weak acids have stronger conjugate bases: Weak acids only partially dissociate, leaving behind a conjugate base that has a greater tendency to accept a proton.
Examples of Conjugate Acid-Base Pairs
Here are a few more examples to solidify your understanding:
| Acid | Conjugate Base |
|---|---|
| HCl (Hydrochloric acid) | Cl⁻ (Chloride ion) |
| HNO₃ (Nitric acid) | NO₃⁻ (Nitrate ion) |
| CH₃COOH (Acetic acid) | CH₃COO⁻ (Acetate ion) |
| NH₄⁺ (Ammonium ion) | NH₃ (Ammonia) |
| H₂CO₃ (Carbonic acid) | HCO₃⁻ (Bicarbonate ion) |
Practical Applications of Conjugate Bases
The concept of conjugate bases has widespread applications in various fields:
- Medicine: Many drugs and biological molecules act as acids or bases, and understanding their conjugate forms is critical for understanding their function and interactions within the body.
- Environmental science: Acid rain and its effects on the environment involve acid-base reactions and the role of conjugate bases in neutralizing acidic pollutants.
- Industrial chemistry: Many industrial processes involve acid-base reactions, and controlling pH is crucial for optimizing these processes. The use of buffer solutions, which rely on conjugate acid-base pairs, is widespread.
- Analytical chemistry: Titration, a common analytical technique, relies heavily on the principles of acid-base chemistry and the use of indicators that change color at specific pH values, reflecting the interaction of conjugate acids and bases.
Conclusion: Mastering the Concept of Conjugate Bases
Understanding the conjugate base of HSO₄⁻, and the broader concept of conjugate acid-base pairs, is a cornerstone of acid-base chemistry. This knowledge is essential for comprehending numerous chemical processes, predicting reaction outcomes, and performing calculations related to pH and equilibrium. By mastering this fundamental concept, you unlock a deeper understanding of the intricate world of acid-base reactions and their significant role in various scientific disciplines and everyday life. Remember the key relationship: a stronger acid will have a weaker conjugate base, and vice versa. This inverse relationship is crucial for predicting the behavior of acids and bases in solution. The amphoteric nature of some species, like HSO₄⁻, further highlights the complexity and dynamism of acid-base chemistry, requiring a nuanced approach to understanding their behavior in different contexts.
Latest Posts
Latest Posts
-
What Is The Function Of Areolar Tissue
Mar 28, 2025
-
What Is 6 25 As A Fraction
Mar 28, 2025
-
Which Of The Following Compounds Is Ionic
Mar 28, 2025
-
Which Characteristic Is Common To All Chordates
Mar 28, 2025
-
Give The Major Product For The Following Reaction
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is Conjugate Base Of Hso4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
