What Is An Ion With A Positive Charge Called
News Leon
Mar 26, 2025 · 5 min read
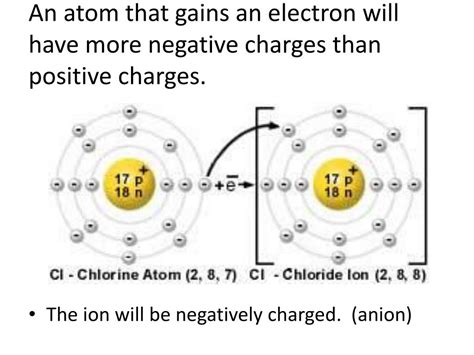
Table of Contents
- What Is An Ion With A Positive Charge Called
- Table of Contents
- What is an Ion with a Positive Charge Called? A Deep Dive into Cations
- Formation of Cations: The Loss of Electrons
- The Role of Electronegativity and Ionization Energy
- Examples of Cation Formation:
- Naming Cations: Simple and Systematic Approaches
- Properties of Cations: Size, Charge Density, and Reactivity
- Significance of Cations in Different Fields:
- 1. Biology:
- 2. Chemistry:
- 3. Physics:
- 4. Geology:
- Common Examples of Cations:
- Conclusion: The Ubiquitous Role of Cations
- Latest Posts
- Latest Posts
- Related Post
What is an Ion with a Positive Charge Called? A Deep Dive into Cations
An ion with a positive charge is called a cation. This seemingly simple definition opens the door to a fascinating world of chemistry, physics, and biology. Understanding cations is crucial for comprehending numerous natural processes and technological advancements. This article will explore cations in detail, covering their formation, properties, naming conventions, examples, and their significance across various scientific disciplines.
Formation of Cations: The Loss of Electrons
At the heart of cation formation lies the loss of one or more electrons. Atoms, in their neutral state, possess an equal number of protons (positively charged particles in the nucleus) and electrons (negatively charged particles orbiting the nucleus). When an atom loses electrons, the number of protons exceeds the number of electrons, resulting in a net positive charge. This positively charged atom is now a cation.
The Role of Electronegativity and Ionization Energy
The tendency of an atom to lose electrons and form a cation is influenced by two key factors:
-
Electronegativity: This refers to an atom's ability to attract electrons towards itself. Atoms with low electronegativity are more likely to lose electrons and form cations. These are generally found on the left side of the periodic table (alkali and alkaline earth metals).
-
Ionization Energy: This is the energy required to remove an electron from a neutral atom. Atoms with low ionization energy readily lose electrons, making cation formation energetically favorable. Again, this correlates with the position of the element on the periodic table.
Examples of Cation Formation:
Let's consider the formation of a sodium cation (Na⁺):
A neutral sodium atom has 11 protons and 11 electrons. It readily loses one electron from its outermost shell to achieve a stable electron configuration (like that of neon), resulting in a cation with 11 protons and 10 electrons. The net positive charge is +1.
Similarly, a magnesium atom (Mg) loses two electrons to form a Mg²⁺ cation, achieving a stable octet.
Naming Cations: Simple and Systematic Approaches
Naming cations follows relatively straightforward rules:
-
Monatomic Cations (single atom cations): The name of the cation is simply the name of the element followed by the word "ion" and its charge as a roman numeral in parenthesis. For example, Na⁺ is a sodium(I) ion, while Mg²⁺ is a magnesium(II) ion. Sometimes, for common cations, the Roman numeral is omitted. For instance, Na+ is often referred to simply as a sodium ion.
-
Polyatomic Cations (cations composed of multiple atoms): These have specific names that are typically memorized. For example, NH₄⁺ is called the ammonium ion.
Properties of Cations: Size, Charge Density, and Reactivity
Cations exhibit several important properties:
-
Smaller Size: Compared to their neutral parent atoms, cations are generally smaller. This is because the loss of electrons reduces electron-electron repulsion, allowing the remaining electrons to be drawn closer to the nucleus.
-
Charge Density: This refers to the ratio of the cation's charge to its size. Smaller cations with higher charges have higher charge densities, making them more reactive.
-
Reactivity: Cations are generally reactive, particularly those with higher charge densities. They tend to participate in chemical reactions by attracting and bonding with anions (negatively charged ions).
Significance of Cations in Different Fields:
Cations play vital roles across various scientific disciplines:
1. Biology:
-
Electrolyte Balance: Cations like sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and magnesium (Mg²⁺) are essential electrolytes in biological systems. They regulate fluid balance, nerve impulses, muscle contractions, and enzyme activity. Imbalances in cation concentrations can lead to serious health problems.
-
Bone Structure: Calcium ions (Ca²⁺) are critical components of bones and teeth, providing structural integrity.
-
Enzyme Function: Many enzymes require specific cations as cofactors to function correctly. These cations often participate in the catalytic mechanism of the enzyme.
2. Chemistry:
-
Ionic Compounds: Cations are fundamental building blocks of ionic compounds, where they are electrostatically attracted to anions to form stable crystalline structures. Examples include sodium chloride (NaCl – table salt), calcium carbonate (CaCO₃ – limestone), and magnesium oxide (MgO).
-
Redox Reactions: Cations are often involved in redox (reduction-oxidation) reactions, where electrons are transferred between chemical species.
-
Catalysis: Certain cations act as catalysts in various chemical reactions, accelerating reaction rates without being consumed in the process.
3. Physics:
-
Semiconductors: Doping semiconductors with cations can alter their electrical conductivity, creating materials with specific electronic properties vital in transistors, integrated circuits, and other electronic devices.
-
Electrochemistry: Cations play a key role in electrochemical processes, such as batteries and fuel cells, where the flow of ions drives the generation of electricity.
4. Geology:
-
Mineral Formation: Cations are crucial for the formation of numerous minerals found in rocks and soils. Their specific combinations and crystal structures determine the mineral's properties.
-
Soil Chemistry: The availability of cations in soil influences plant growth and nutrient uptake.
Common Examples of Cations:
Here's a table summarizing some common cations, their charges, and their sources or significance:
| Cation | Symbol | Charge | Significance |
|---|---|---|---|
| Sodium ion | Na⁺ | +1 | Electrolyte, nerve impulses, table salt |
| Potassium ion | K⁺ | +1 | Electrolyte, nerve impulses |
| Calcium ion | Ca²⁺ | +2 | Bone structure, muscle contraction, electrolyte |
| Magnesium ion | Mg²⁺ | +2 | Enzyme cofactor, electrolyte |
| Ammonium ion | NH₄⁺ | +1 | Fertilizer, acid-base chemistry |
| Iron(II) ion | Fe²⁺ | +2 | Hemoglobin, biological processes |
| Iron(III) ion | Fe³⁺ | +3 | Hemoglobin, biological processes |
| Copper(II) ion | Cu²⁺ | +2 | Enzyme cofactor, biological processes |
| Zinc ion | Zn²⁺ | +2 | Enzyme cofactor, biological processes |
Conclusion: The Ubiquitous Role of Cations
Cations are fundamental building blocks of matter, playing crucial roles in a vast array of natural and technological processes. Their properties, determined by their size, charge, and electronic configuration, dictate their behavior and influence their interactions with other ions and molecules. From the intricate workings of biological systems to the development of advanced materials, understanding cations is essential for advancing our knowledge and developing innovative technologies. The seemingly simple question of "what is an ion with a positive charge called?" thus leads to a surprisingly rich and complex exploration of the chemical and physical world.
Latest Posts
Latest Posts
-
Is Baking Soda An Element Compound Or Mixture
Mar 27, 2025
-
A Kilobyte Is Equal To Approximately One Bytes
Mar 27, 2025
-
What Is The Physical Appearance Of An Organism
Mar 27, 2025
-
Does Denser Medium Have Higher Refractive Index
Mar 27, 2025
-
The Proposal By Anton Chekhov Summary
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Is An Ion With A Positive Charge Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
