Is Baking Soda An Element Compound Or Mixture
News Leon
Mar 27, 2025 · 5 min read
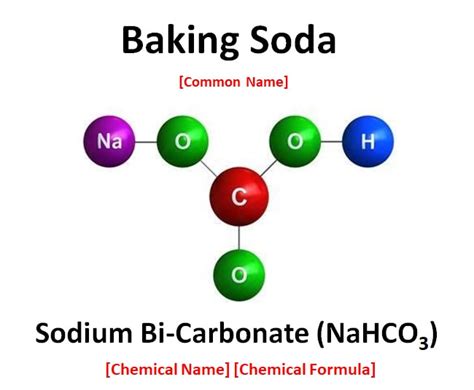
Table of Contents
Is Baking Soda an Element, Compound, or Mixture? A Deep Dive into Sodium Bicarbonate
Baking soda, a ubiquitous kitchen staple, is more than just a leavening agent; it's a fascinating chemical substance with a rich history and diverse applications. Understanding its fundamental nature – whether it's an element, compound, or mixture – is crucial to appreciating its properties and uses. This comprehensive guide delves into the chemical composition of baking soda, differentiating it from elements and mixtures, and exploring its unique characteristics.
Understanding the Three Categories: Element, Compound, and Mixture
Before we classify baking soda, let's define each category:
Elements:
Elements are the fundamental building blocks of matter. They are substances that cannot be broken down into simpler substances by chemical means. Each element is defined by its unique number of protons in its atomic nucleus, represented by its atomic number on the periodic table. Examples include hydrogen (H), oxygen (O), and sodium (Na). Elements are pure substances consisting of only one type of atom.
Compounds:
Compounds are pure substances formed when two or more different elements chemically combine in a fixed ratio. This chemical bonding creates a new substance with properties distinct from its constituent elements. The atoms in a compound are held together by strong chemical bonds, such as ionic or covalent bonds. Water (H₂O), for example, is a compound formed from the elements hydrogen and oxygen. The properties of water are very different from the properties of hydrogen and oxygen gases.
Mixtures:
Mixtures are combinations of two or more substances that are not chemically bonded. They can be homogeneous (uniform throughout, like saltwater) or heterogeneous (non-uniform, like sand and water). The components of a mixture retain their individual properties, and the proportions of the components can vary. Unlike compounds, mixtures can be separated into their constituent substances using physical methods, such as filtration or evaporation.
Baking Soda: A Chemical Compound
Baking soda, also known as sodium bicarbonate (NaHCO₃), is unequivocally a compound. It's formed from the chemical combination of three elements:
- Sodium (Na): An alkali metal, highly reactive and soft.
- Hydrogen (H): The lightest element, a highly flammable gas.
- Carbon (C): A non-metal, the basis of organic chemistry.
- Oxygen (O): A highly reactive non-metal, essential for respiration.
These elements are chemically bonded together in a specific ratio to form sodium bicarbonate. The strong ionic and covalent bonds within the molecule determine its unique physical and chemical properties, such as its alkalinity and its ability to release carbon dioxide when heated or reacted with an acid. You cannot simply separate sodium, hydrogen, carbon, and oxygen from baking soda through simple physical means. Chemical reactions are required.
Distinguishing Baking Soda from Elements and Mixtures
Let's compare baking soda to elements and mixtures to further solidify its classification:
Baking Soda vs. Elements: Baking soda is clearly different from elements because it's composed of more than one type of atom. It contains sodium, hydrogen, carbon, and oxygen atoms bonded together in a specific arrangement. Elements, on the other hand, consist of only one type of atom. You cannot break down baking soda into its constituent elements without employing chemical processes.
Baking Soda vs. Mixtures: Baking soda is distinct from mixtures because its components are chemically bonded, not merely physically mixed. The properties of baking soda are not a simple sum of the properties of its constituent elements. The ratio of these elements is fixed and consistent in pure baking soda, whereas the composition of a mixture can vary widely. You can't separate the elements of baking soda through simple physical processes like filtration or distillation; chemical processes are needed.
The Chemical Structure and Properties of Sodium Bicarbonate
Understanding the chemical structure of sodium bicarbonate is vital in appreciating its unique properties. The compound consists of a sodium cation (Na⁺) and a bicarbonate anion (HCO₃⁻). The bicarbonate anion is interesting because it contains a carbon atom bonded to three oxygen atoms, one of which is also bonded to a hydrogen atom. This arrangement results in resonance structures, influencing the chemical reactivity of the bicarbonate ion.
The properties stemming from this structure include:
- Alkalinity: Baking soda is a weak base, meaning it can neutralize acids. This property is what makes it useful in baking, where it reacts with acidic ingredients to produce carbon dioxide gas, causing dough to rise.
- Carbon Dioxide Release: When heated or mixed with an acid, sodium bicarbonate decomposes, releasing carbon dioxide gas (CO₂), water (H₂O), and sodium carbonate (Na₂CO₃). This reaction is fundamental to its use as a leavening agent in baking and as a fire suppressant.
- Solubility: Baking soda is soluble in water, allowing it to be easily dissolved and incorporated into various solutions and mixtures.
- Mild Abrasiveness: Its slightly abrasive nature makes it useful as a cleaning agent, helping to scrub away dirt and stains.
Practical Applications Leveraging Baking Soda's Compound Nature
The unique chemical properties of baking soda, stemming directly from its nature as a compound, lead to its versatile applications in many fields:
- Baking: As a leavening agent, baking soda reacts with acidic ingredients in recipes (like buttermilk or vinegar) to create carbon dioxide bubbles, making cakes, cookies, and other baked goods rise.
- Cleaning: Its mild abrasiveness and alkalinity make it an effective cleaning agent for removing stains, grease, and odors from various surfaces.
- Deodorizing: It neutralizes odors by absorbing and reacting with odor-causing molecules.
- Fire Suppression: Baking soda is used in some fire extinguishers because it can react with and neutralize acids produced during combustion, suppressing the flames.
- Medicine: It is sometimes used as an antacid to relieve heartburn due to its ability to neutralize stomach acid. (Always consult a doctor before using baking soda for medicinal purposes.)
- Personal Care: It can be used as an ingredient in toothpaste, deodorants, and other personal care products.
Conclusion: Baking Soda – A Powerful Compound
In conclusion, baking soda (sodium bicarbonate, NaHCO₃) is definitively a chemical compound, not an element or a mixture. Its unique properties, derived from the specific chemical bonds between sodium, hydrogen, carbon, and oxygen atoms, make it a remarkably versatile substance with numerous applications in baking, cleaning, medicine, and other fields. Understanding its fundamental chemical nature helps us appreciate its capabilities and utilize its potential effectively and safely. Its consistent chemical composition, unlike mixtures, allows for predictable and reliable results in its various applications. The intricate interplay of its constituent elements highlights the wonders of chemical bonding and the remarkable diversity found within chemical compounds.
Latest Posts
Latest Posts
-
Which Of The Following Is An Aromatic Hydrocarbon
Mar 30, 2025
-
Which Of The Following Statements Is Incorrect About Estrogen
Mar 30, 2025
-
The Composition Of Lymph Is Most Similar To
Mar 30, 2025
-
Which Statement Describes The Law Of Independent Assortment
Mar 30, 2025
-
Circumference Of A Circle With A Radius Of 3
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Is Baking Soda An Element Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
