What Is A Zero Dipole Moment
News Leon
Mar 24, 2025 · 6 min read
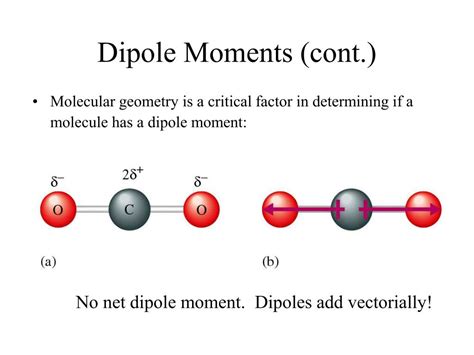
Table of Contents
What is a Zero Dipole Moment? Understanding Molecular Polarity and Symmetry
Understanding molecular polarity is crucial in chemistry, impacting various properties like boiling point, solubility, and reactivity. A key concept within this field is the dipole moment, a measure of the molecule's overall polarity. This article delves into the fascinating world of zero dipole moments, explaining what they are, how they arise, and their implications. We'll explore the relationship between molecular geometry, bond polarity, and the resulting dipole moment, providing clear examples and illustrations to solidify your understanding.
What is a Dipole Moment?
Before understanding zero dipole moments, we need to grasp the concept of a dipole moment itself. A dipole moment (µ) arises when there's an uneven distribution of electron density within a molecule. This happens when there's a difference in electronegativity between the atoms involved in a bond. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond.
When two atoms with different electronegativities bond, the more electronegative atom pulls the shared electrons closer, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the less electronegative atom. This separation of charge forms an electric dipole, similar to a tiny magnet with a positive and negative pole. The dipole moment is a vector quantity, meaning it has both magnitude (size) and direction. The magnitude is proportional to the charge separation and the distance between the charges.
The dipole moment is usually expressed in Debye (D) units, where 1 D = 3.336 × 10⁻³⁰ C·m (Coulomb-meter). A larger dipole moment indicates a greater degree of charge separation and hence, higher polarity.
The Significance of Molecular Geometry
The overall dipole moment of a molecule isn't simply the sum of individual bond dipoles. Molecular geometry plays a crucial role. Even if individual bonds are polar, the molecule as a whole might have a zero dipole moment due to the symmetrical arrangement of the bonds. This cancellation of bond dipoles is the key to understanding zero dipole moments.
Symmetrical Molecules: The Key to Zero Dipole Moments
Imagine a molecule with two identical polar bonds arranged symmetrically. The bond dipoles will point in opposite directions, effectively canceling each other out. The resultant dipole moment of the molecule will be zero. This is the hallmark of molecules with zero dipole moments: their geometry allows for the complete cancellation of individual bond dipoles.
Examples of Molecules with Zero Dipole Moments
Several molecular structures naturally lead to zero dipole moments. Let's examine some prominent examples:
1. Carbon Dioxide (CO₂)
Carbon dioxide is a linear molecule with two oxygen atoms bonded to a central carbon atom. Each C=O bond is polar, with oxygen being more electronegative than carbon. However, because the molecule is linear, the two bond dipoles are equal in magnitude and point in opposite directions, resulting in a net dipole moment of zero.
Visual Representation:
O=C=O
← → (Bond dipoles cancel)
2. Methane (CH₄)
Methane has a tetrahedral geometry with four C-H bonds. While each C-H bond possesses a small dipole moment (carbon is slightly more electronegative than hydrogen), the symmetrical tetrahedral arrangement ensures that these bond dipoles cancel each other out perfectly. Therefore, methane has a zero dipole moment.
Visual Representation: (Imagine a tetrahedron with C at the center and H at each vertex)
3. Benzene (C₆H₆)
Benzene is a planar, hexagonal molecule with alternating single and double bonds. While individual C-C and C-H bonds have dipole moments, the high symmetry of the molecule leads to a net dipole moment of zero. The bond dipoles effectively cancel each other out due to the symmetrical arrangement of atoms.
Visual Representation: (Imagine a regular hexagon with alternating single and double bonds)
4. Tetrachloromethane (CCl₄)
Tetrachloromethane (carbon tetrachloride) has a tetrahedral geometry similar to methane. However, the C-Cl bonds are more polar than C-H bonds because chlorine is much more electronegative than hydrogen. Despite the greater polarity of individual bonds, the symmetrical arrangement still leads to a net dipole moment of zero.
Visual Representation: (Imagine a tetrahedron with C at the center and Cl at each vertex)
Implications of Zero Dipole Moment
The absence of a dipole moment has significant implications for the physical and chemical properties of a molecule:
-
Solubility: Nonpolar molecules with zero dipole moments tend to be insoluble in polar solvents (like water) but soluble in nonpolar solvents (like hexane). This is because "like dissolves like."
-
Boiling Point: Generally, molecules with zero dipole moments have lower boiling points compared to polar molecules of similar size and molecular weight. This is because the intermolecular forces (van der Waals forces) are weaker in nonpolar molecules.
-
Reactivity: The reactivity of a molecule is influenced by its polarity. Nonpolar molecules with zero dipole moments may have different reactivity patterns compared to polar molecules. They may react preferentially with nonpolar reagents.
Determining Dipole Moment: Experimental and Theoretical Approaches
The dipole moment of a molecule can be determined experimentally using techniques like microwave spectroscopy or dielectric constant measurements. These methods provide direct measurements of the molecular dipole moment.
Theoretical calculations, using computational chemistry techniques like Density Functional Theory (DFT), can also predict dipole moments. These calculations rely on models of the electronic structure and provide estimates of the dipole moment. While not as precise as experimental measurements, theoretical calculations offer valuable insights, especially for complex molecules.
Beyond Simple Molecules: More Complex Cases
While the examples provided focus on relatively simple molecules, the principles of symmetry and bond dipole cancellation apply to more complex molecules as well. Many larger organic molecules, such as symmetrical alkanes and certain aromatic compounds, also exhibit zero dipole moments due to their symmetrical structures.
However, subtle variations in geometry or the presence of functional groups can disrupt the symmetry and lead to a non-zero dipole moment. For instance, the addition of a polar functional group to a symmetrical molecule can alter its dipole moment significantly.
Conclusion: Understanding the Implications of Molecular Polarity
Understanding the concept of a zero dipole moment is crucial for grasping the fundamental principles of molecular polarity and its influence on the properties and reactivity of molecules. The symmetrical arrangement of atoms and the cancellation of individual bond dipoles are key factors determining whether a molecule possesses a zero dipole moment or not. This knowledge is not only valuable for students of chemistry but also critical for researchers in various fields, including materials science, drug design, and environmental chemistry. By understanding molecular polarity, we gain valuable insight into the behavior and interactions of molecules at a fundamental level. This understanding allows us to predict the properties and behavior of molecules, ultimately leading to a deeper comprehension of the natural world.
Latest Posts
Latest Posts
-
No Mans Sky 1 2 6 24
Mar 26, 2025
-
Which Of The Following Will Show Tyndall Effect Salt Solution
Mar 26, 2025
-
What Is The Boiling Point On The Fahrenheit Scale
Mar 26, 2025
-
Which Of The Following Connects Muscle To Bone
Mar 26, 2025
-
Is An Amoeba Eukaryotic Or Prokaryotic
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is A Zero Dipole Moment . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
