Which Of The Following Will Show Tyndall Effect Salt Solution
News Leon
Mar 26, 2025 · 5 min read
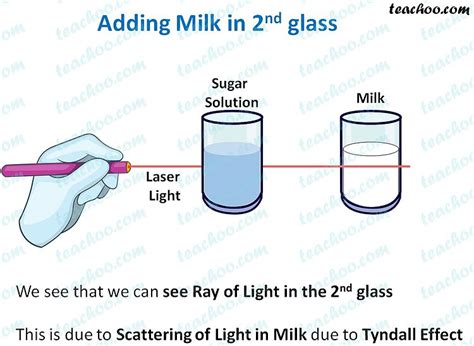
Table of Contents
Which of the following will show Tyndall effect: Salt Solution? Unveiling the Mysteries of Colloidal Dispersions
The Tyndall effect, a fascinating optical phenomenon, illuminates the difference between true solutions and colloids. This article delves deep into the nature of the Tyndall effect, exploring its underlying principles and explaining why certain solutions, like salt solutions, exhibit different behaviors when a beam of light is passed through them. We'll investigate various types of mixtures and their interactions with light to definitively answer the question: Will a salt solution show the Tyndall effect?
Understanding the Tyndall Effect: A Light Scattering Phenomenon
The Tyndall effect is the scattering of light as a light beam passes through a colloid. A colloid is a heterogeneous mixture containing particles that are larger than those in a true solution but smaller than those in a suspension. These particles, typically ranging in size from 1 to 1000 nanometers, are dispersed throughout the medium. When a beam of light encounters these particles, it's scattered in various directions, making the beam visible. This is in contrast to a true solution, where the solute particles are too small to scatter light noticeably, resulting in an invisible light beam's passage.
Key characteristics of the Tyndall effect:
- Scattering of light: The light beam is scattered in all directions, making it visible.
- Wavelength dependence: The intensity of scattering is inversely proportional to the fourth power of the wavelength (λ). This means shorter wavelengths (e.g., blue light) are scattered more strongly than longer wavelengths (e.g., red light). This is why the scattered light often appears bluish.
- Particle size: The effect is only observable in colloids, where the particle size is in the appropriate range (1-1000 nm).
True Solutions vs. Colloids: A Comparative Analysis
To understand why the Tyndall effect is observed in some solutions and not others, it’s crucial to differentiate between true solutions and colloidal dispersions.
True Solutions:
- Particle size: Solute particles are extremely small, typically less than 1 nanometer in diameter. They are dissolved at the molecular or ionic level.
- Homogeneity: The mixture is completely homogeneous; the solute particles are evenly distributed throughout the solvent.
- Light scattering: No visible scattering of light occurs. The light beam passes through undisturbed. Examples include salt dissolved in water, sugar dissolved in water, and many other solutions of small molecules.
Colloids:
- Particle size: Particles are larger than in true solutions, ranging from 1 to 1000 nanometers. These particles can be individual molecules, aggregates of molecules, or tiny droplets of liquid.
- Heterogeneity: Although appearing homogeneous to the naked eye, colloids are actually heterogeneous mixtures. The dispersed particles are not uniformly dissolved but remain suspended.
- Light scattering: They exhibit the Tyndall effect, scattering light and making the beam visible. Examples include milk, fog, ink, and blood.
Salt Solution and the Tyndall Effect: A Definitive Answer
Now, let's address the central question: Does a salt solution (e.g., NaCl in water) show the Tyndall effect?
The answer is no. A salt solution, such as sodium chloride dissolved in water, is a true solution. When NaCl dissolves in water, it dissociates into sodium (Na⁺) and chloride (Cl⁻) ions. These ions are extremely small, far smaller than the 1-nanometer threshold required for light scattering. As such, they do not scatter visible light. When a beam of light is passed through a salt solution, it passes through unaffected, remaining invisible.
Other Examples of Mixtures and their Tyndall Effect Behavior
Let's examine other mixtures to further illustrate the principles of light scattering and the Tyndall effect:
Mixtures exhibiting the Tyndall Effect:
- Milk: Milk is an emulsion, a type of colloid, with tiny fat globules dispersed in water. The fat globules scatter light, resulting in a visible beam.
- Fog: Fog consists of tiny water droplets suspended in air. These droplets scatter light, making the beam visible.
- Ink: Many inks contain colloidal particles that scatter light.
- Blood: Blood is a complex colloid with various particles suspended in plasma, leading to light scattering.
- Gelatin: Gelatin solutions are colloids where gelatin molecules form a network throughout the solution. This network can scatter light, exhibiting the Tyndall effect.
Mixtures not exhibiting the Tyndall Effect:
- Sugar water: Sugar dissolves completely in water, forming a true solution. The sugar molecules are too small to scatter light.
- Alcohol in water: Alcohol and water are miscible, forming a homogeneous mixture. The molecules are too small to scatter light.
- Air (generally): Air itself doesn't usually display the Tyndall effect unless there's significant particulate matter like dust or smoke present.
Applications of the Tyndall Effect
The Tyndall effect has various practical applications, including:
- Distinguishing between true solutions and colloids: As discussed, the presence or absence of the Tyndall effect is a simple test to differentiate these two types of mixtures.
- Atmospheric science: The scattering of light in the atmosphere explains the blue color of the sky and the red color of sunsets.
- Aerosol detection: The Tyndall effect is used to detect the presence of aerosols in the atmosphere.
- Medical diagnosis: The Tyndall effect can be used in medical diagnoses, for example, in the analysis of blood samples.
Conclusion: The Tyndall Effect and its Relevance to Mixture Classification
Understanding the Tyndall effect is crucial for characterizing the nature of different mixtures. The size of the dispersed particles plays a critical role in determining whether a mixture will exhibit light scattering. As we've shown conclusively, a salt solution, being a true solution with extremely small ions, does not display the Tyndall effect. The absence of light scattering provides strong evidence for the homogeneous nature of this type of solution at the molecular level. By understanding the principles behind the Tyndall effect and the differences between true solutions and colloids, we can better appreciate the complex world of mixtures and their interactions with light. This knowledge holds significance in diverse fields, from atmospheric science and environmental monitoring to medical diagnostics and material science.
Latest Posts
Latest Posts
-
Which Of The Following Is Bacteriostatic
Mar 29, 2025
-
How Many Unpaired Electrons Does Cobalt Have
Mar 29, 2025
-
Which Of The Following Is Not Output Device
Mar 29, 2025
-
Which Of The Following Bodies Has The Largest Kinetic Energy
Mar 29, 2025
-
A Cell That Contains One Set Of Chromosomes
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Will Show Tyndall Effect Salt Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
