How Many Unpaired Electrons Does Cobalt Have
News Leon
Mar 29, 2025 · 6 min read
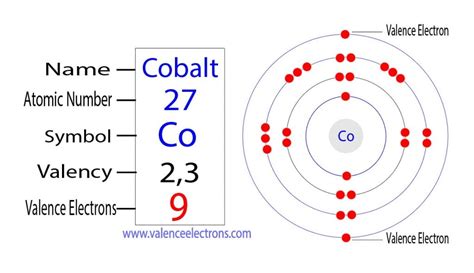
Table of Contents
How Many Unpaired Electrons Does Cobalt Have? A Deep Dive into Electronic Configuration and Magnetic Properties
Cobalt, a transition metal with a fascinating array of applications, possesses a unique electronic structure that dictates its magnetic properties and chemical behavior. Understanding the number of unpaired electrons in cobalt is crucial to grasping its role in various fields, from catalysis to medicine. This article delves deep into the electronic configuration of cobalt, exploring the factors influencing the number of unpaired electrons and highlighting the implications of this characteristic.
The Electronic Configuration of Cobalt: The Foundation of Unpaired Electrons
Cobalt, with an atomic number of 27, boasts a complex electronic configuration. To determine the number of unpaired electrons, we need to understand its electron arrangement within its orbitals. The standard electronic configuration follows the Aufbau principle and Hund's rule:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁷
This configuration reveals that cobalt has seven electrons in its 3d orbitals. According to Hund's rule, these electrons will individually occupy each of the five 3d orbitals before pairing up. This leads to three unpaired electrons in the ground state of a neutral cobalt atom.
Hund's Rule: The Key to Unpaired Electrons
Hund's rule is fundamental to predicting the number of unpaired electrons. It states that electrons will individually occupy each orbital within a subshell before pairing up. This is due to the lower energy state associated with maximizing spin multiplicity. Each electron possesses a spin (either +1/2 or -1/2), and parallel spins (all +1/2 or all -1/2) within a subshell result in a lower energy configuration than paired spins (+1/2 and -1/2). Therefore, cobalt's 3d orbitals are populated with three unpaired electrons and two paired electrons in its ground state.
Influence of Oxidation State: A Variable Number of Unpaired Electrons
The number of unpaired electrons in cobalt is not fixed; it's highly dependent on its oxidation state. Cobalt exhibits a range of oxidation states, most commonly +2 and +3, but also others including +1, +4, and even higher oxidation states in specific circumstances. The oxidation state dramatically influences the electronic configuration and, consequently, the number of unpaired electrons.
Cobalt(II) (Co²⁺): Maintaining Unpaired Electrons
When cobalt loses two electrons to become Co²⁺, these electrons are typically removed from the 4s orbital. This leaves the 3d orbitals largely unchanged, resulting in:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁷
Thus, Co²⁺ still possesses three unpaired electrons. This is significant for the magnetic properties of cobalt(II) compounds, making them paramagnetic.
Cobalt(III) (Co³⁺): A Shift in the Unpaired Electron Count
In the case of Co³⁺, three electrons are removed. The most likely scenario is the removal of two electrons from the 4s orbital and one from a 3d orbital, leading to:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁶
Following Hund's rule, this results in four unpaired electrons in Co³⁺. The change in the number of unpaired electrons from Co²⁺ to Co³⁺ highlights the significant influence of oxidation state on the electronic structure.
Ligand Field Effects: The Impact of Coordination Chemistry
The presence of ligands, molecules or ions that bind to the central metal ion, significantly affects the electronic configuration and the number of unpaired electrons in coordination complexes of cobalt. The ligand field theory provides a framework to understand these effects. Ligands create a ligand field that splits the d-orbitals into different energy levels. The magnitude of this splitting is dependent on the nature of the ligands (strong-field or weak-field ligands).
Strong-Field Ligands: Pairing Up Electrons
Strong-field ligands cause a large splitting of the d-orbitals. This can lead to pairing of electrons to occupy the lower energy orbitals before filling the higher energy orbitals. This phenomenon can reduce the number of unpaired electrons, even in cases where the oxidation state might suggest otherwise. In some cobalt(II) complexes with strong-field ligands, pairing might reduce the number of unpaired electrons to one or even zero, resulting in diamagnetism or low paramagnetism.
Weak-Field Ligands: Maintaining or Increasing Unpaired Electrons
Weak-field ligands result in a smaller energy gap between the split d-orbitals. In such complexes, electrons will preferentially occupy individual orbitals before pairing, maximizing spin multiplicity, as dictated by Hund's rule. This often leads to a higher number of unpaired electrons compared to complexes with strong-field ligands. Some cobalt(II) complexes with weak-field ligands might maintain the three unpaired electrons, while others could even show higher numbers due to intricate electronic interactions.
Implications of Unpaired Electrons: Magnetic Properties and Reactivity
The number of unpaired electrons directly impacts the magnetic properties and chemical reactivity of cobalt compounds.
Paramagnetism and Magnetic Applications
The presence of unpaired electrons makes cobalt compounds paramagnetic. This means they are attracted to magnetic fields. This property is crucial in various applications, including:
- Magnetic recording materials: Cobalt alloys are widely used in magnetic recording media due to their high coercivity and remanence, properties directly linked to their unpaired electrons.
- Magnetic resonance imaging (MRI): Cobalt complexes are being explored as contrast agents in MRI due to their paramagnetic properties.
- Catalysis: The paramagnetic nature of cobalt catalysts can influence their catalytic activity.
Reactivity and Catalytic Activity
The unpaired electrons in cobalt also influence its reactivity and catalytic activity. The presence of unpaired electrons allows for easier participation in redox reactions, making cobalt an effective catalyst in various chemical processes.
- Vitamin B12: Cobalt is a crucial component of vitamin B12 (cobalamin), where its variable oxidation states and unpaired electrons play a critical role in its biological functions.
- Industrial catalysis: Cobalt is a vital component in many industrial catalysts used in processes like hydroformylation, Fischer-Tropsch synthesis, and other reactions where its ability to readily accept and donate electrons is essential.
Beyond the Basics: Complexities and Exceptions
While the basic principles outlined above provide a good understanding of the number of unpaired electrons in cobalt, it's crucial to acknowledge complexities and exceptions:
- Excited states: At higher energy levels, electron configurations can deviate from the ground state, influencing the number of unpaired electrons.
- Ligand field effects: The precise number of unpaired electrons in cobalt complexes is highly sensitive to the specific ligands present and their arrangement. Advanced computational methods are often required for accurate predictions in complex coordination environments.
- Relativistic effects: In heavier elements, relativistic effects can become significant and influence the electronic structure and properties, adding another layer of complexity.
Conclusion: A Dynamic Metal with Variable Unpaired Electron Count
The number of unpaired electrons in cobalt is not a static value. It's a dynamic property that varies depending on the oxidation state, the presence of ligands, and other factors. Understanding these influences is crucial for comprehending the diverse applications of this versatile metal. From the magnetic properties exploited in technology to the catalytic activity underpinning crucial chemical reactions and biological functions, the unpaired electrons in cobalt are central to its importance across various scientific disciplines. Further research continues to unravel the subtleties of cobalt's electronic structure, deepening our understanding of this fascinating element.
Latest Posts
Latest Posts
-
What Cell Organelle Does Photosynthesis Occur
Mar 31, 2025
-
How Many Isotopes Does Fluorine Have
Mar 31, 2025
-
Refraction Causes The Bottom Of A Swimming Pool To Appear
Mar 31, 2025
-
Glucagon Stimulates Glycogenolysis In The Liver True Or False
Mar 31, 2025
-
Classify The Following Mixtures As Heterogeneous Or Homogeneous
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Unpaired Electrons Does Cobalt Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
