What Is The Boiling Point On The Fahrenheit Scale
News Leon
Mar 26, 2025 · 5 min read
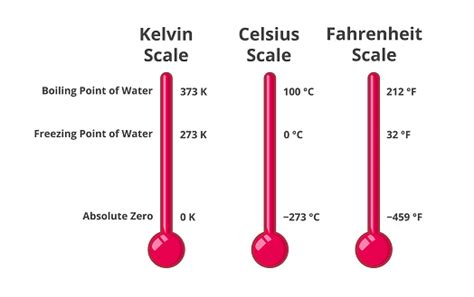
Table of Contents
What is the Boiling Point on the Fahrenheit Scale? A Deep Dive into Temperature Measurement
Understanding temperature is fundamental to numerous scientific disciplines, culinary practices, and everyday life. While the Celsius scale is widely adopted internationally, the Fahrenheit scale remains prevalent in several countries, particularly the United States. This comprehensive guide delves into the boiling point on the Fahrenheit scale, exploring its significance, historical context, conversion methods, and practical applications.
Understanding the Fahrenheit Scale
The Fahrenheit scale, developed by Daniel Gabriel Fahrenheit in 1724, is a temperature scale where the freezing point of water is 32 degrees Fahrenheit (°F), and the boiling point of water is 212 °F at standard atmospheric pressure. This contrasts with the Celsius scale, where these points are 0 °C and 100 °C, respectively. The scale's increments are smaller than those in Celsius, leading to larger numerical values for the same temperature.
The Significance of 212°F
The boiling point of water at 212°F is not merely a numerical value; it represents a crucial physical phenomenon. At this temperature, water transitions from its liquid state to its gaseous state, a process driven by the increased kinetic energy of its molecules overcoming the intermolecular forces holding them together. This phase transition is vital in various processes, from cooking and industrial applications to meteorological phenomena.
Historical Context and Development
Fahrenheit's original scale was based on three reference points: the temperature of a mixture of ice, water, and ammonium chloride (0 °F), the temperature of ice and water (32 °F), and the temperature of the human body (96 °F). Over time, the scale has been refined, with the boiling point of water at standard atmospheric pressure (101.325 kPa) becoming a key reference point, establishing 212°F. While his original method was somewhat arbitrary, the Fahrenheit scale's adoption reflects its early widespread use and the inertia of established systems.
The Physics Behind Boiling Point
The boiling point of a substance is the temperature at which its vapor pressure equals the surrounding atmospheric pressure. This means that at 212°F and standard atmospheric pressure, the water molecules have enough energy to overcome the pressure of the surrounding air and escape into the gaseous phase, forming steam.
Factors Affecting the Boiling Point
Several factors can affect the boiling point of water and other substances, including:
- Atmospheric Pressure: Higher atmospheric pressure requires a higher temperature for water to boil because the molecules need more energy to overcome the greater external pressure. At higher altitudes, where atmospheric pressure is lower, water boils at a lower temperature (below 212°F).
- Impurities: Dissolving substances in water can alter its boiling point; some solutes raise the boiling point (boiling point elevation), while others can slightly lower it. This principle is exploited in various applications, including cooking and industrial processes.
- Altitude: As previously mentioned, altitude significantly influences boiling point. At higher elevations, the lower atmospheric pressure allows water to boil at temperatures below 212°F. This affects cooking times and requires adjustments in recipes.
Practical Applications of the Boiling Point
The boiling point of water at 212°F is crucial in numerous everyday and industrial applications:
Cooking and Food Preparation
Cooking relies heavily on the boiling point of water. Boiling water is used to cook pasta, vegetables, and other foods, utilizing the heat transfer and the chemical changes that occur at this temperature. Different cooking methods leverage varying temperatures to achieve desired textures and flavors. Understanding the impact of altitude on the boiling point is critical for adjusting cooking times and methods at higher elevations.
Industrial Processes
Numerous industrial processes depend on boiling water or steam. Steam generation for power plants, sterilization techniques in medicine and food processing, and various chemical reactions utilize the heat and phase transition of water at its boiling point. Steam is employed for cleaning, heating, and driving turbines, highlighting the importance of this fundamental physical property.
Meteorology and Climate Science
Understanding the boiling point of water is critical in meteorology and climate science. The water cycle, involving evaporation, condensation, and precipitation, is heavily influenced by temperature and pressure, with the boiling point acting as a key threshold. The study of clouds, weather patterns, and climate modeling necessitates a thorough grasp of phase transitions and the factors influencing them.
Converting Between Fahrenheit and Celsius
While the Fahrenheit scale is still used in certain contexts, the Celsius scale is the preferred standard in most scientific and international settings. It is essential to be able to convert between these scales.
The formulas for converting between Fahrenheit (°F) and Celsius (°C) are:
- Fahrenheit to Celsius: °C = (°F - 32) × 5/9
- Celsius to Fahrenheit: °F = (°C × 9/5) + 32
Using these formulas, we can easily convert the boiling point of water:
- 212°F to Celsius: (212 - 32) × 5/9 = 100°C
This confirms that the boiling point of water is 100°C on the Celsius scale.
Beyond Water: Boiling Points of Other Substances
While this article focuses on the boiling point of water, it's important to remember that every substance has its own unique boiling point, influenced by factors like molecular structure, intermolecular forces, and pressure. For example, ethanol boils at 173.1°F (78.4°C), while mercury boils at 674.9°F (357.0°C). These variations highlight the complexity of phase transitions and the diverse applications of boiling point in different scientific and industrial fields.
Conclusion: The Enduring Significance of 212°F
The boiling point of water at 212°F on the Fahrenheit scale is far more than a simple number; it's a cornerstone of our understanding of physical science, impacting various aspects of our daily lives and industrial processes. From cooking a meal to generating power, the phase transition of water at this critical temperature drives numerous fundamental applications. Understanding this concept, along with the ability to convert between temperature scales, provides a foundational understanding of the world around us. The enduring relevance of the Fahrenheit scale, particularly in specific regions, underscores the importance of continuing to understand and utilize its unique characteristics within their respective contexts. Further exploration into the specific applications of 212°F in different fields will reveal its continuing significance in our world.
Latest Posts
Latest Posts
-
How Many Unpaired Electrons Does Cobalt Have
Mar 29, 2025
-
Which Of The Following Is Not Output Device
Mar 29, 2025
-
Which Of The Following Bodies Has The Largest Kinetic Energy
Mar 29, 2025
-
A Cell That Contains One Set Of Chromosomes
Mar 29, 2025
-
An Electric Vehicle Starts From Rest And Accelerates
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Boiling Point On The Fahrenheit Scale . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
